The EndoPredict Gene-Expression Assay in Clinical Practice - Performance and Impact on Clinical Decisions
- PMID: 23826382
- PMCID: PMC3694878
- DOI: 10.1371/journal.pone.0068252
The EndoPredict Gene-Expression Assay in Clinical Practice - Performance and Impact on Clinical Decisions
Abstract
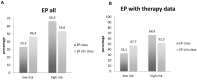
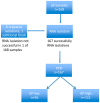
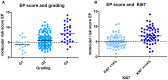
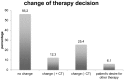
The validated EndoPredict assay is a novel tool to predict the risk of metastases of patients with estrogen receptor positive, HER2 negative breast cancer treated with endocrine therapy alone. It has been designed to integrate genomic and clinical information and includes clinico-pathological factors such as tumor size and nodal status. The test is feasible in a decentral setting in molecular pathology laboratories. In this project, we investigated the performance of this test in clinical practice, and performed a retrospective evaluation of its impact on treatment decisions in breast cancer. During one year, EndoPredict assays from 167 patients could be successfully performed. For retrospective evaluation of treatment decisions, a questionnaire was sent to the clinical partner. Regarding the molecular EP class, samples from 56 patients (33.5%) had a low-risk, whereas 111 patients (66.5%) showed a high-risk gene profile. After integration of the clinicopathological factors the combined clinical and molecular score (EPclin) resulted in a low-risk group of 77 patients (46.4%), while 89 (53.6%) had a high risk EPclin score. The EPclin-based estimated median 10-year-risk for metastases with endocrine therapy alone was 11% for the whole cohort. The median handling time averaged three days (range: 0 to 11 days), 59.3% of the tests could be performed in three or less than three days. Comparison of pre- and post-test therapy decisions showed a change of therapy in 37.7% of patients. 16 patients (12.3%) had a change to an additional chemotherapy while 25.4% of patients (n = 33) changed to an endocrine therapy alone. In 73 patients (56.2%) no change of therapy resulted. In 6.1% of patients (n = 8), the patients did not agree to the recommendation of the tumor board. Our results show that the EndoPredict assay could be routinely performed in decentral molecular pathology laboratories and the results markedly change treatment decisions.
Conflict of interest statement
Figures

References
-
- Siegel R, Naishadham D, Jemal A (2013) Cancer statistics. CA Cancer J Clin 63: 11–30. - PubMed
-
- Oakman C, Santarpia L, Di Leo A (2010) Breast cancer assessment tools and optimizing adjuvant therapy. Nat Rev Clin Oncol 7: 725–732. - PubMed
-
- Aebi S, Davidson T, Gruber G, Castiglione M (2011) Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21 Suppl 5v9–14. - PubMed
-
- Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63: 181–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

