Aerobic exercise combined with antioxidative treatment does not counteract moderate- or mid-stage Alzheimer-like pathophysiology of APP/PS1 mice
- PMID: 23827013
- PMCID: PMC6493399
- DOI: 10.1111/cns.12139
Aerobic exercise combined with antioxidative treatment does not counteract moderate- or mid-stage Alzheimer-like pathophysiology of APP/PS1 mice
Abstract
Aims: The present study evaluated the combined treatment effects of aerobic exercise and antioxidative stress on moderate-stage Alzheimer's disease (AD).
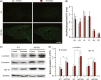
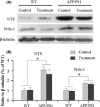
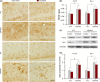
Methods: Ten-month-old APP/PS1 mice were given antioxidative treatment with acetylcysteine, along with aerobic exercise for 6 weeks. Spatial learning and memory were tested using the Morris water maze, and β-amyloid (Aβ) plaque deposits in the forebrain were quantified by Thioflavin-S staining. Levels of soluble Aβ1-42, β-secretase enzyme, ү-secretase enzyme, oxidative and antioxidant stress markers nitrotyrosine and peroxiredoxin-1, glial markers glial fibrillary acidic protein and ionized calcium-binding adaptor molecule 1, and synaptic protein synaptophysin in the hippocampus were all measured by western blotting and/or immunohistochemistry.
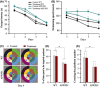
Results: APP/PS1 mice showed severe declines in spatial learning and memory compared with their wild-type littermates, which were not attenuated by aerobic exercise combined with antioxidative treatment. The pathologic analysis revealed that Aβ deposition and production, oxidative stress, glial inflammation, and synaptic loss were not mitigated in the brain of exercised APP/PS1 mice, compared with the sedentary APP/PS1 animals.
Conclusion: This study reveals that a combined treatment of aerobic exercise plus antioxidative stress does not counteract pathophysiology in the moderate- or mid-stages of AD.
Keywords: APP/PS1 mice; Aerobic exercise; Alzheimer's disease; Antioxidative treatment; Cognitive function; Pathology; β-amyloid.
© 2013 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Aerobic Exercise Ameliorates Alzheimer's Disease-Like Pathology by Regulating Hepatic Phagocytosis of Aβ.Front Biosci (Landmark Ed). 2025 Apr 22;30(4):36597. doi: 10.31083/FBL36597. Front Biosci (Landmark Ed). 2025. PMID: 40302344
-
Treadmill exercise enhances synaptic plasticity, but does not alter β-amyloid deposition in hippocampi of aged APP/PS1 transgenic mice.Neuroscience. 2015 Jul 9;298:357-66. doi: 10.1016/j.neuroscience.2015.04.038. Epub 2015 Apr 23. Neuroscience. 2015. PMID: 25917310
-
Four-month treadmill exercise prevents the decline in spatial learning and memory abilities and the loss of spinophilin-immunoreactive puncta in the hippocampus of APP/PS1 transgenic mice.Neurobiol Dis. 2020 Mar;136:104723. doi: 10.1016/j.nbd.2019.104723. Epub 2019 Dec 27. Neurobiol Dis. 2020. PMID: 31887353
-
Benzo(a)pyrene exposure induced neuronal loss, plaque deposition, and cognitive decline in APP/PS1 mice.J Neuroinflammation. 2020 Aug 31;17(1):258. doi: 10.1186/s12974-020-01925-y. J Neuroinflammation. 2020. PMID: 32867800 Free PMC article.
-
CART mitigates oxidative stress and DNA damage in memory deficits of APP/PS1 mice via upregulating β‑amyloid metabolism‑associated enzymes.Mol Med Rep. 2021 Apr;23(4):280. doi: 10.3892/mmr.2021.11919. Epub 2021 Feb 19. Mol Med Rep. 2021. PMID: 33604684 Free PMC article.
Cited by
-
Qualitative Evaluation Informs Understanding of Motor Cognition and Therapies in Older Adults with Mild Cognitive Impairment.J Alzheimers Dis. 2021;84(2):691-703. doi: 10.3233/JAD-210617. J Alzheimers Dis. 2021. PMID: 34569954 Free PMC article.
-
Age-dependent shift in the de novo proteome accompanies pathogenesis in an Alzheimer's disease mouse model.Commun Biol. 2021 Jun 30;4(1):823. doi: 10.1038/s42003-021-02324-6. Commun Biol. 2021. PMID: 34193971 Free PMC article.
-
Alzheimer's Disease: From Pathogenesis to Mesenchymal Stem Cell Therapy - Bridging the Missing Link.Front Cell Neurosci. 2022 Feb 7;15:811852. doi: 10.3389/fncel.2021.811852. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35197824 Free PMC article. Review.
-
Treadmill Exercise Decreases Aβ Deposition and Counteracts Cognitive Decline in APP/PS1 Mice, Possibly via Hippocampal Microglia Modifications.Front Aging Neurosci. 2019 Apr 5;11:78. doi: 10.3389/fnagi.2019.00078. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31024293 Free PMC article.
-
Voluntary running does not reduce neuroinflammation or improve non-cognitive behavior in the 5xFAD mouse model of Alzheimer's disease.Sci Rep. 2020 Jan 28;10(1):1346. doi: 10.1038/s41598-020-58309-8. Sci Rep. 2020. PMID: 31992814 Free PMC article.
References
-
- Cummings JL. Alzheimer's disease. N Engl J Med 2004;351:56–67. - PubMed
-
- Ferrer I. Defining Alzheimer as a common age‐related neurodegenerative process not inevitably leading to dementia. Prog Neurobiol 2012;97:38–51. - PubMed
-
- Mullane K, Williams M. Alzheimer's therapeutics: Continued clinical failures question the validity of the amyloid hypothesis‐but what lies beyond? Biochem Pharmacol 2013;85:289–305. - PubMed
-
- Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 2005;99:1193–1204. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

