Pathogenicity of porcine G9P[23] and G9P[7] rotaviruses in piglets
- PMID: 23827353
- PMCID: PMC7117468
- DOI: 10.1016/j.vetmic.2013.05.024
Pathogenicity of porcine G9P[23] and G9P[7] rotaviruses in piglets
Abstract
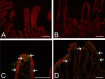
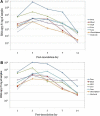
G9 group A rotaviruses (RVAs) are considered important pathogens in pigs and humans, and pigs are hypothesized to be a potential host reservoir for human. However, intestinal and extra-intestinal pathogenicity and viremia of porcine G9 RVAs has remained largely unreported. In this study, colostrum-deprived piglets were orally infected with a porcine G9P[23] or G9P[7] strain. Histopathologically, both strains induced characteristic small intestinal lesions. Degeneration and necrosis of parenchymal cells were observed in the extra-intestinal tissues, but most predominantly in the mesenteric lymph nodes (MLNs). RVA antigen was continuously detected in the small intestinal mucosa and MLNs, but only transiently in cells of the liver, lung, and choroid plexus. Viral RNA levels were much higher in the feces and the MLNs compared to other tissues. The onset of viremia occurred at day post infection (DPI) 1 with the amount of viral RNA reaching its peak at DPI 3 or 5, before decreasing significantly at DPI 7 and remaining detectable until DPI 14. Our data suggest that porcine G9 RVAs have a strong small intestinal tropism, are highly virulent for piglets, have the ability to escape the small intestine, spread systemically via viremia, and replicate in extra-intestinal tissues. In addition, MLNs might act as a secondary site for viral amplification and the portal of systemic entry. These results add to our understanding of the pathogenesis of human G9 RVAs, and the validity of the pig model for use with both human and pig G9 RVAs in further studies.
Keywords: Experimental model; G9 genotype; Group A rotaviruses; Pathogenicity; Piglets.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures




References
-
- Barril P.A., Martinez L.C., Giordano M.O., Castello A.A., Rota R.P., Isa M.B., Masachessi G., Ferreyra L.J., Glikmann G., Nates S.V. Detection of group a human rotavirus G9 genotype circulating in Córdoba, Argentina, as early as 1980. J. Med. Virol. 2006;78:1113–1118. - PubMed
-
- Blutt S.E., Conner M.E. Rotavirus: to the gut and beyond! Curr. Opin. Gastroenterol. 2007;23:39–43. - PubMed
-
- Blutt S.E., Kirkwood C.D., Parreño V., Warfield K.L., Ciarlet M., Estes M.K., Bok K., Bishop R.F., Conner M.E. Rotavirus antigenaemia and viraemia: a common event? Lancet. 2003;362:1445–1449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

