Distinct α-synuclein strains differentially promote tau inclusions in neurons
- PMID: 23827677
- PMCID: PMC3820001
- DOI: 10.1016/j.cell.2013.05.057
Distinct α-synuclein strains differentially promote tau inclusions in neurons
Abstract
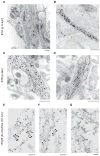
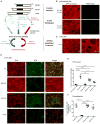
Many neurodegenerative diseases are characterized by the accumulation of insoluble protein aggregates, including neurofibrillary tangles comprised of tau in Alzheimer's disease and Lewy bodies composed of α-synuclein in Parkinson's disease. Moreover, different pathological proteins frequently codeposit in disease brains. To test whether aggregated α-synuclein can directly cross-seed tau fibrillization, we administered preformed α-synuclein fibrils assembled from recombinant protein to primary neurons and transgenic mice. Remarkably, we discovered two distinct strains of synthetic α-synuclein fibrils that demonstrated striking differences in the efficiency of cross-seeding tau aggregation, both in neuron cultures and in vivo. Proteinase K digestion revealed conformational differences between the two synthetic α-synuclein strains and also between sarkosyl-insoluble α-synuclein extracted from two subgroups of Parkinson's disease brains. We speculate that distinct strains of pathological α-synuclein likely exist in neurodegenerative disease brains and may underlie the tremendous heterogeneity of synucleinopathies.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Comment in
-
Reaping what you sow: Cross-seeding between aggregation-prone proteins in neurodegeneration.Mov Disord. 2014 Mar;29(3):306. doi: 10.1002/mds.25766. Epub 2014 Jan 2. Mov Disord. 2014. PMID: 24395732 No abstract available.
References
-
- Abeliovich A, Schmitz Y, Fariñas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Aguzzi A, Heikenwalder M, Polymenidou M. Insights into prion strains and neurotoxicity. Nat Rev Mol Cell Biol. 2007;8:552–561. - PubMed
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. - PubMed
-
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

