The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling
- PMID: 23827710
- PMCID: PMC3703100
- DOI: 10.1016/j.stem.2013.05.003
The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling
Abstract
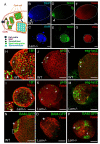
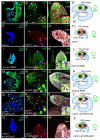
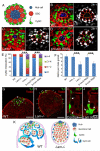
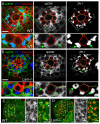
Stem cell niche interactions have been studied extensively with regard to cell polarity and extracellular signaling. Less is known about the way in which signals and polarity cues integrate with intracellular structures to ensure appropriate niche organization and function. Here, we report that nuclear lamins function in the cyst stem cells (CySCs) of Drosophila testes to control the interaction of CySCs with the hub. This interaction is important for regulation of CySC differentiation and organization of the niche that supports the germline stem cells (GSCs). Lamin promotes nuclear retention of phosphorylated ERK in the CySC lineage by regulating the distribution of specific nucleoporins within the nuclear pores. Lamin-regulated nuclear epidermal growth factor (EGF) receptor signaling in the CySC lineage is essential for proliferation and differentiation of the GSCs and the transient amplifying germ cells. Thus, we have uncovered a role for the nuclear lamina in the integration of EGF signaling to regulate stem cell niche function.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
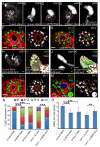
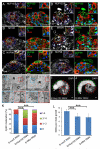
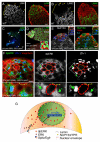
References
-
- Al-Haboubi T, Shumaker DK, Koser J, Wehnert M, Fahrenkrog B. Distinct association of the nuclear pore protein Nup153 with A- and B-type lamins. Nucleus. 2011;2:500–509. - PubMed
-
- Bank EM, Gruenbaum Y. The nuclear lamina and heterochromatin: a complex relationship. Biochem Soc Trans. 2011;39:1705–1709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

