The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation
- PMID: 23827711
- PMCID: PMC3767186
- DOI: 10.1016/j.stem.2013.06.003
The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation
Abstract
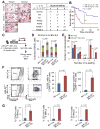
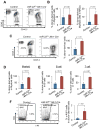
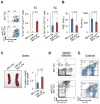
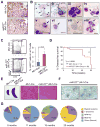
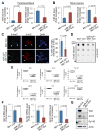
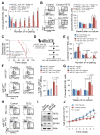
MicroRNAs are frequently deregulated in cancer. Here we show that miR-22 is upregulated in myelodysplastic syndrome (MDS) and leukemia and its aberrant expression correlates with poor survival. To explore its role in hematopoietic stem cell function and malignancy, we generated transgenic mice conditionally expressing miR-22 in the hematopoietic compartment. These mice displayed reduced levels of global 5-hydroxymethylcytosine (5-hmC) and increased hematopoietic stem cell self-renewal accompanied by defective differentiation. Conversely, miR-22 inhibition blocked proliferation in both mouse and human leukemic cells. Over time, miR-22 transgenic mice developed MDS and hematological malignancies. We also identify TET2 as a key target of miR-22 in this context. Ectopic expression of TET2 suppressed the miR-22-induced phenotypes. Downregulation of TET2 protein also correlated with poor clinical outcomes and miR-22 overexpression in MDS patients. Our results therefore identify miR-22 as a potent proto-oncogene and suggest that aberrations in the miR-22/TET2 regulatory network are common in hematopoietic malignancies.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

Comment in
-
Oncogenic miRNAs and the perils of losing control of a stem cell's epigenetic identity.Cell Stem Cell. 2013 Jul 3;13(1):5-6. doi: 10.1016/j.stem.2013.06.012. Cell Stem Cell. 2013. PMID: 23827702
References
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

