SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors
- PMID: 23827712
- PMCID: PMC3736853
- DOI: 10.1016/j.stem.2013.05.014
SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors
Abstract
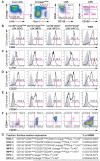
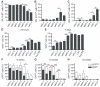
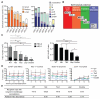
Hematopoietic stem cells (HSCs) and multipotent hematopoietic progenitors (MPPs) are routinely isolated using various markers but remain heterogeneous. Here we show that four SLAM family markers, CD150, CD48, CD229, and CD244, can distinguish HSCs and MPPs from restricted progenitors and subdivide them into a hierarchy of functionally distinct subpopulations with stepwise changes in cell-cycle status, self-renewal, and reconstituting potential. CD229 expression largely distinguished lymphoid-biased HSCs from rarely dividing myeloid-biased HSCs, enabling prospective enrichment of these HSC subsets. Differences in CD229 and CD244 expression resolved CD150(-)CD48(-/low)Lineage(-/low)Sca-1(+)c-Kit(+) cells into a hierarchy of highly purified MPPs that retained erythroid and platelet potential but exhibited progressive changes in mitotic activity and reconstituting potential. Use of these markers, and reconstitution assays, showed that conditional deletion of Scf from endothelial cells and perivascular stromal cells eliminated the vast majority of bone marrow HSCs, including nearly all CD229(-/low) HSCs, demonstrating that quiescent HSCs are maintained by a perivascular niche.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




References
-
- Adolfsson J, Mansson R, Buza-Vidas N, Hultquist A, Liuba K, Jensen CT, Bryder D, Yang L, Borge OJ, Thoren LA, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. - PubMed
-
- Benveniste P, Frelin C, Janmohamed S, Barbara M, Herrington R, Hyam D, Iscove NN. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell. 2010;6:48–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

