Pannexin 1 involvement in bladder dysfunction in a multiple sclerosis model
- PMID: 23827947
- PMCID: PMC3701900
- DOI: 10.1038/srep02152
Pannexin 1 involvement in bladder dysfunction in a multiple sclerosis model
Abstract
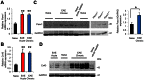
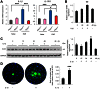
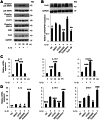
Bladder dysfunction is common in Multiple Sclerosis (MS) but little is known of its pathophysiology. We show that mice with experimental autoimmune encephalomyelitis (EAE), a MS model, have micturition dysfunction and altered expression of genes associated with bladder mechanosensory, transduction and signaling systems including pannexin 1 (Panx1) and Gja1 (encoding connexin43, referred to here as Cx43). EAE mice with Panx1 depletion (Panx1(-/-)) displayed similar neurological deficits but lesser micturition dysfunction compared to Panx1(+/+) EAE. Cx43 and IL-1β upregulation in Panx1(+/+) EAE bladder mucosa was not observed in Panx1(-/-) EAE. In urothelial cells, IL-1β stimulation increased Cx43 expression, dye-coupling, and p38 MAPK phosphorylation but not ERK1/2 phosphorylation. SB203580 (p38 MAPK inhibitor) prevented IL-1β-induced Cx43 upregulation. IL-1β also increased IL-1β, IL-1R-1, PANX1 and CASP1 expression. Mefloquine (Panx1 blocker) reduced these IL-1β responses. We propose that Panx1 signaling provides a positive feedback loop for inflammatory responses involved in bladder dysfunction in MS.
Figures




References
-
- McCombe P. A., Gordon T. P. & Jackson M. W. Bladder dysfunction in multiple sclerosis. Expert Rev. Neurother. 9, 331–340 (2009). - PubMed
-
- de Seze M., Ruffion A., Denys P., Joseph P. A. & Perrouin-Verbe B. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult. Scler. 13, 915–928 (2007). - PubMed
-
- Lassmann H. Models of multiple sclerosis: new insights into pathophysiology and repair. Curr. Opin. Neurol. 21, 242–247 (2008). - PubMed
-
- Al-Izki S., Pryce G., Giovannoni G. & Baker D. Evaluating potential therapies for bladder dysfunction in a mouse model of multiple sclerosis with high-resolution ultrasonography. Mult. Scler. 15, 795–801 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

