Strategies for profiling native S-nitrosylation
- PMID: 23828013
- PMCID: PMC4280024
- DOI: 10.1002/bip.22342
Strategies for profiling native S-nitrosylation
Abstract
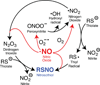
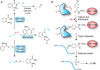
Cysteine is a uniquely reactive amino acid, capable of undergoing both nucleophlilic and oxidative post-translational modifications. One such oxidation reaction involves the covalent modification of cysteine via the gaseous second messenger nitric oxide (NO), termed S-nitrosylation (SNO). This dynamic post-translational modification is involved in the redox regulation of proteins across all phylogenic kingdoms. In mammals, calcium-dependent activation of NO synthase triggers the local release of NO, which activates nearby guanylyl cyclases and cGMP-dependent pathways. In parallel, diffusible NO can locally modify redox active cellular thiols, functionally modulating many redox sensitive enzymes. Aberrant SNO is implicated in the pathology of many diseases, including neurodegeneration, inflammation, and stroke. In this review, we discuss current methods to label sites of SNO for biochemical analysis. The most popular method involves a series of biochemical steps to mask free thiols followed by selective nitrosothiol reduction and capture. Other emerging methods include mechanism-based phosphine probes and mercury enrichment chemistry. By bridging new enrichment approaches with high-resolution mass spectrometry, large-scale analysis of protein nitrosylation has highlighted new pathways of oxidative regulation.
Keywords: nitric oxide; nitrosylation; post-translational modification.
Copyright © 2013 Wiley Periodicals, Inc.
Figures

References
-
- Antoine M, Gand A, Boschi-Muller S, Branlant G. Journal of Biological Chemistry. 2006;281:39062–39070. - PubMed
-
- Jao S-C, Ospina English SM, Berdis AJ, Starke DW, Post CB, Mieyal J. J Biochemistry. 2006;45:4785–4796. - PubMed
-
- Gan ZR, Polokoff MA, Jacobs JW, Sardana MK. Biochemical and Biophysical Research Communications. 1990;168:944–951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

