Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures
- PMID: 23828203
- PMCID: PMC3701895
- DOI: 10.1038/srep02141
Generation of a functional liver tissue mimic using adipose stromal vascular fraction cell-derived vasculatures
Abstract
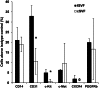
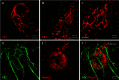
One of the major challenges in cell implantation therapies is to promote integration of the microcirculation between the implanted cells and the host. We used adipose-derived stromal vascular fraction (SVF) cells to vascularize a human liver cell (HepG2) implant. We hypothesized that the SVF cells would form a functional microcirculation via vascular assembly and inosculation with the host vasculature. Initially, we assessed the extent and character of neovasculatures formed by freshly isolated and cultured SVF cells and found that freshly isolated cells have a higher vascularization potential. Generation of a 3D implant containing fresh SVF and HepG2 cells formed a tissue in which HepG2 cells were entwined with a network of microvessels. Implanted HepG2 cells sequestered labeled LDL delivered by systemic intravascular injection only in SVF-vascularized implants demonstrating that SVF cell-derived vasculatures can effectively integrate with host vessels and interface with parenchymal cells to form a functional tissue mimic.
Figures



Similar articles
-
Concentration-Dependent Vascularization of Adipose Stromal Vascular Fraction Cells.Cell Transplant. 2015;24(10):2029-39. doi: 10.3727/096368914X685401. Epub 2014 Nov 13. Cell Transplant. 2015. PMID: 25397993
-
Ectopic osteogenic capacity of freshly isolated adipose-derived stromal vascular fraction cells supported with platelet-rich plasma: A simulation of intraoperative procedure.J Craniomaxillofac Surg. 2016 Oct;44(10):1750-1760. doi: 10.1016/j.jcms.2016.08.011. Epub 2016 Aug 24. J Craniomaxillofac Surg. 2016. PMID: 27624644
-
Engineering adipose tissue from uncultured human adipose stromal vascular fraction on collagen matrix and gelatin sponge scaffolds.Tissue Eng Part A. 2011 Jun;17(11-12):1489-98. doi: 10.1089/ten.TEA.2010.0688. Epub 2011 Mar 4. Tissue Eng Part A. 2011. PMID: 21247363
-
Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature.J Plast Reconstr Aesthet Surg. 2016 Feb;69(2):170-9. doi: 10.1016/j.bjps.2015.10.015. Epub 2015 Oct 31. J Plast Reconstr Aesthet Surg. 2016. PMID: 26565755 Review.
-
Adipose-Derived Stromal Vascular Fraction Cells: Update on Clinical Utility and Efficacy.Crit Rev Eukaryot Gene Expr. 2015;25(2):145-52. doi: 10.1615/critreveukaryotgeneexpr.2015013057. Crit Rev Eukaryot Gene Expr. 2015. PMID: 26080608 Review.
Cited by
-
A review of cellularization strategies for tissue engineering of whole organs.Front Bioeng Biotechnol. 2015 Mar 30;3:43. doi: 10.3389/fbioe.2015.00043. eCollection 2015. Front Bioeng Biotechnol. 2015. PMID: 25870857 Free PMC article. Review.
-
Effect of Stromal Vascular Fractions on Angiogenesis of Injected Diced Cartilage.J Craniofac Surg. 2022 Mar-Apr 01;33(2):713-718. doi: 10.1097/SCS.0000000000007996. J Craniofac Surg. 2022. PMID: 35013075 Free PMC article.
-
Stromal Vascular Fraction Reverses the Age-Related Impairment in Revascularization following Injury.J Vasc Res. 2022;59(6):343-357. doi: 10.1159/000526002. Epub 2022 Sep 8. J Vasc Res. 2022. PMID: 36075199 Free PMC article.
-
Stromal Cells Promote Neovascular Invasion Across Tissue Interfaces.Front Physiol. 2020 Aug 14;11:1026. doi: 10.3389/fphys.2020.01026. eCollection 2020. Front Physiol. 2020. PMID: 33013445 Free PMC article.
-
Women's contribution to stem cell research for osteoarthritis: an opinion paper.Front Cell Dev Biol. 2023 Dec 19;11:1209047. doi: 10.3389/fcell.2023.1209047. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38174070 Free PMC article. Review. No abstract available.
References
-
- Fox I. J. & Roy-Chowdhury J. Hepatocyte transplantation. J. Hepatol. 40, 878–886 (2004). - PubMed
-
- Strom S. C. et al. Transplantation of human hepatocytes. Transplant. Proc. 29, 2103–2106 (1997). - PubMed
-
- Habibullah C. M., Syed I. H., Qamar A. & Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation 58, 951–952 (1994). - PubMed
-
- Nagata H. et al. Prolonged survival of porcine hepatocytes in cynomolgus monkeys. Gastroenterology 132, 321–329 (2007). - PubMed
-
- Kaully T., Kaufman-Francis K., Lesman A. & Levenberg S. Vascularization--the conduit to viable engineered tissues. Tissue Eng. Part B Rev. 15, 159–169 (2009). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

