Manumycin A corrects aberrant splicing of Clcn1 in myotonic dystrophy type 1 (DM1) mice
- PMID: 23828222
- PMCID: PMC3701899
- DOI: 10.1038/srep02142
Manumycin A corrects aberrant splicing of Clcn1 in myotonic dystrophy type 1 (DM1) mice
Abstract
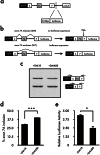
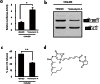
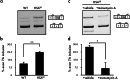
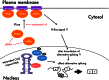
Myotonic dystrophy type 1 (DM1) is the most common muscular dystrophy in adults and as yet no cure for DM1. Here, we report the potential of manumycin A for a novel DM1 therapeutic reagent. DM1 is caused by expansion of CTG repeat. Mutant transcripts containing expanded CUG repeats lead to aberrant regulation of alternative splicing. Myotonia (delayed muscle relaxation) is the most commonly observed symptom in DM1 patients and is caused by aberrant splicing of the skeletal muscle chloride channel (CLCN1) gene. Identification of small-molecule compounds that correct aberrant splicing in DM1 is attracting much attention as a way of improving understanding of the mechanism of DM1 pathology and improving treatment of DM1 patients. In this study, we generated a reporter screening system and searched for small-molecule compounds. We found that manumycin A corrects aberrant splicing of Clcn1 in cell and mouse models of DM1.
Figures

Similar articles
-
[Myotonic dystrophy].Rinsho Shinkeigaku. 2013;53(11):1109-11. doi: 10.5692/clinicalneurol.53.1109. Rinsho Shinkeigaku. 2013. PMID: 24291894 Review. Japanese.
-
Muscle chloride channel dysfunction in two mouse models of myotonic dystrophy.J Gen Physiol. 2007 Jan;129(1):79-94. doi: 10.1085/jgp.200609635. Epub 2006 Dec 11. J Gen Physiol. 2007. PMID: 17158949 Free PMC article.
-
The Dimeric Form of 1,3-Diaminoisoquinoline Derivative Rescued the Mis-splicing of Atp2a1 and Clcn1 Genes in Myotonic Dystrophy Type 1 Mouse Model.Chemistry. 2020 Nov 11;26(63):14305-14309. doi: 10.1002/chem.202001572. Epub 2020 Oct 6. Chemistry. 2020. PMID: 32449537 Free PMC article.
-
Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing.Mol Cell. 2002 Jul;10(1):45-53. doi: 10.1016/s1097-2765(02)00572-5. Mol Cell. 2002. PMID: 12150906
-
Myotonic dystrophy: emerging mechanisms for DM1 and DM2.Biochim Biophys Acta. 2007 Feb;1772(2):195-204. doi: 10.1016/j.bbadis.2006.05.013. Epub 2006 Jun 20. Biochim Biophys Acta. 2007. PMID: 16876389 Review.
Cited by
-
In Vitro and In Vivo Modulation of Alternative Splicing by the Biguanide Metformin.Mol Ther Nucleic Acids. 2015 Nov 3;4(11):e262. doi: 10.1038/mtna.2015.35. Mol Ther Nucleic Acids. 2015. PMID: 26528939 Free PMC article.
-
Hybrid splicing minigene and antisense oligonucleotides as efficient tools to determine functional protein/RNA interactions.Sci Rep. 2017 Dec 14;7(1):17587. doi: 10.1038/s41598-017-17816-x. Sci Rep. 2017. PMID: 29242583 Free PMC article.
-
Small Molecules Which Improve Pathogenesis of Myotonic Dystrophy Type 1.Front Neurol. 2018 May 18;9:349. doi: 10.3389/fneur.2018.00349. eCollection 2018. Front Neurol. 2018. PMID: 29867749 Free PMC article. Review.
-
Mitigating RNA Toxicity in Myotonic Dystrophy using Small Molecules.Int J Mol Sci. 2019 Aug 17;20(16):4017. doi: 10.3390/ijms20164017. Int J Mol Sci. 2019. PMID: 31426500 Free PMC article. Review.
-
RNA Splicing and Disease: Animal Models to Therapies.Trends Genet. 2019 Jan;35(1):68-87. doi: 10.1016/j.tig.2018.10.002. Epub 2018 Nov 19. Trends Genet. 2019. PMID: 30466729 Free PMC article. Review.
References
-
- Harper P. S. Myotonic Dystophy, third edn. (2001).
-
- Ranum L. P. & Cooper T. A. RNA-mediated neuromuscular disorders. Annu Rev Neurosci 29, 259–277 (2006). - PubMed
-
- Aslanidis C. et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature 355, (1992). - PubMed
-
- Brook J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 69, 385 (1992). - PubMed
-
- Liquori C. L. et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293, 864–867 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

