High-mobility group box 2 is associated with prognosis of glioblastoma by promoting cell viability, invasion, and chemotherapeutic resistance
- PMID: 23828241
- PMCID: PMC3748920
- DOI: 10.1093/neuonc/not078
High-mobility group box 2 is associated with prognosis of glioblastoma by promoting cell viability, invasion, and chemotherapeutic resistance
Abstract
Background: The expression profile of high-mobility group box 2 (HMGB2) in patients with glioblastoma multiforme (GBM) and its clinical signature with underlying mechanisms were not fully explored.
Methods: HMGB2 protein levels were measured in 51 GBM patients by immunohistochemical studies. To clarify the precise role of HMGB2 on cell invasion and viability of 3 GBM cell lines, we did in vitro and in vivo analyses with lentivirus vectors and small interfering RNA. Transwell invasion assays and wound-healing assays were used to analyze the invasion of GBM cells. Expression of p53 and matrix metalloproteinase 2/tissue inhibitors of metalloproteinase 2 (MMP2/TIMP2) protein was analyzed by Western blot.
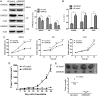
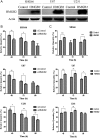
Results: HMGB2 protein expression was significantly higher in GBM than in controlled brain tissues (P < .0001). HMGB2 overexpression was significantly correlated with shorter overall survival time, which was the only independent prognostic factor for overall survival in a multivariate analysis (P = .017). HMGB2 knockdown by small interfering RNA decreased cell viability and invasion in vitro and significantly decreased tumor volume in vivo, which might be involved in the change of p53 expression and the balance of MMP2/TIMP2. Moreover, silencing of HMGB2 could significantly increase the sensitivity of GBM cells to temozolomide chemotherapy.
Conclusions: Our present data suggest that HMGB2 expression is a significant prognostic factor and might play an important role in cell invasion and temozolomide-induced chemotherapeutic sensitivity of GBM. This study highlights the importance of HMGB2 as a novel prognostic marker and an attractive therapeutic target of GBM.
Keywords: glioblastoma; high-mobility group box 2; invasion; prognosis; temozolomide.
Figures



Similar articles
-
Calpain suppresses cell growth and invasion of glioblastoma multiforme by producing the cleavage of filamin A.Int J Clin Oncol. 2020 Jun;25(6):1055-1066. doi: 10.1007/s10147-020-01636-7. Epub 2020 Feb 27. Int J Clin Oncol. 2020. PMID: 32103382
-
Annexin A5 promotes invasion and chemoresistance to temozolomide in glioblastoma multiforme cells.Tumour Biol. 2014 Dec;35(12):12327-37. doi: 10.1007/s13277-014-2545-1. Epub 2014 Sep 23. Tumour Biol. 2014. PMID: 25245332
-
High Mobility Group Box 1 (HMGB1) Predicts Invasion and Poor Prognosis of Glioblastoma Multiforme via Activating AKT Signaling in an Autocrine Pathway.Med Sci Monit. 2018 Dec 9;24:8916-8924. doi: 10.12659/MSM.912104. Med Sci Monit. 2018. PMID: 30531692 Free PMC article.
-
Oncogenesis, Microenvironment Modulation and Clinical Potentiality of FAP in Glioblastoma: Lessons Learned from Other Solid Tumors.Cells. 2021 May 10;10(5):1142. doi: 10.3390/cells10051142. Cells. 2021. PMID: 34068501 Free PMC article. Review.
-
The role of IL-17 in the pathogenesis and treatment of glioblastoma-an update on the state of the art and future perspectives.Med Oncol. 2024 Jun 25;41(8):187. doi: 10.1007/s12032-024-02434-1. Med Oncol. 2024. PMID: 38918274 Free PMC article. Review.
Cited by
-
Structure and Functions of HMGB2 Protein.Int J Mol Sci. 2023 May 5;24(9):8334. doi: 10.3390/ijms24098334. Int J Mol Sci. 2023. PMID: 37176041 Free PMC article. Review.
-
Meta-analysis of multi-center transcriptomic profiles and machine learning reveal phospholipase Cβ4 as a Wnt/Ca²+ signaling mediator in glioblastoma immunotherapy.Front Immunol. 2025 Aug 7;16:1610683. doi: 10.3389/fimmu.2025.1610683. eCollection 2025. Front Immunol. 2025. PMID: 40852729 Free PMC article.
-
Glioma-associated antigen HEATR1 induces functional cytotoxic T lymphocytes in patients with glioma.J Immunol Res. 2014;2014:131494. doi: 10.1155/2014/131494. Epub 2014 Jul 9. J Immunol Res. 2014. PMID: 25126583 Free PMC article.
-
Biological functions and theranostic potential of HMGB family members in human cancers.Ther Adv Med Oncol. 2020 Nov 10;12:1758835920970850. doi: 10.1177/1758835920970850. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 33224279 Free PMC article. Review.
-
High level of LncRNA MAPKAPK5-AS1 predicts poor prognosis and contributes to the malignant proliferation and EMT of non-small cell lung cancer via sponging miR-490-3p from HMGB2.Genes Genomics. 2023 May;45(5):611-625. doi: 10.1007/s13258-022-01339-5. Epub 2022 Nov 29. Genes Genomics. 2023. PMID: 36445573
References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

