Therapy-resistant acute lymphoblastic leukemia (ALL) cells inactivate FOXO3 to escape apoptosis induction by TRAIL and Noxa
- PMID: 23828551
- PMCID: PMC3759677
- DOI: 10.18632/oncotarget.953
Therapy-resistant acute lymphoblastic leukemia (ALL) cells inactivate FOXO3 to escape apoptosis induction by TRAIL and Noxa
Abstract
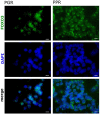
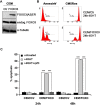
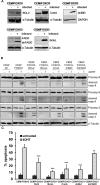
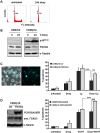
Forkhead transcription factors (FOXO) are downstream targets of the phosphoinositol-3-kinase (PI3K) protein kinase B (PKB) signaling cascade and play a pivotal role in cell differentiation, cell cycle and apoptosis. We found that cells from prednisone-resistant T-acute lymphoblastic leukemia (T-ALL) patients showed cytoplasmic localization of FOXO3 in comparison to prednisone-sensitive patients suggesting its inactivation. To determine the impact of FOXO3, T-ALL cells were infected with a 4OH-tamoxifen-regulated, phosphorylation-independent FOXO3(A3)ERtm allele. After FOXO3-activation these cells undergo caspase-dependent apoptosis. FOXO3 induces the death ligand TRAIL and the BH3-only protein Noxa implicating extrinsic as well as intrinsic death signaling. Whereas dnFADD partially inhibited cell death, CrmA and dnBID efficiently rescued ALL cells after FOXO3 activation, suggesting a caspase-8 amplifying feedback loop downstream of FADD. Knockdown of TRAIL and Noxa reduced FOXO3-induced apoptosis, implicating that mitochondrial destabilization amplifies TRAIL-signaling. The-reconstitution of the cell cycle inhibitor p16INK4A, which sensitizes ALL cells to mitochondria-induced cell death, represses FOXO3 protein levels and reduces the dependency of these leukemia cells on PI3K-PKB signaling. This suggests that if p16INK4A is deleted during leukemia development, FOXO3 levels elevate and FOXO3 has to be inactivated by deregulation of the PI3K-PKB pathway to prevent FOXO3-induced cell death.
Conflict of interest statement
The authors declare no conflict of interest
Figures

Similar articles
-
FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells.Cell Death Differ. 2007 Mar;14(3):534-47. doi: 10.1038/sj.cdd.4402017. Epub 2006 Aug 4. Cell Death Differ. 2007. PMID: 16888645
-
Resistance to TRAIL-induced apoptosis caused by constitutional phosphorylation of Akt and PTEN in acute lymphoblastic leukemia cells.Exp Hematol. 2008 Oct;36(10):1343-53. doi: 10.1016/j.exphem.2008.04.011. Epub 2008 Jul 2. Exp Hematol. 2008. PMID: 18599181
-
Inactivation of the forkhead transcription factor FoxO3 is essential for PKB-mediated survival of hematopoietic progenitor cells by kit ligand.Exp Hematol. 2003 Apr;31(4):316-23. doi: 10.1016/s0301-472x(03)00002-x. Exp Hematol. 2003. PMID: 12691919
-
New insights into Notch1 regulation of the PI3K-AKT-mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia.Cell Signal. 2014 Jan;26(1):149-61. doi: 10.1016/j.cellsig.2013.09.021. Epub 2013 Oct 16. Cell Signal. 2014. PMID: 24140475 Review.
-
Therapeutic Consequences of Targeting the IGF-1/PI3K/AKT/FOXO3 Axis in Sarcopenia: A Narrative Review.Cells. 2023 Dec 7;12(24):2787. doi: 10.3390/cells12242787. Cells. 2023. PMID: 38132107 Free PMC article. Review.
Cited by
-
Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors.Oncotarget. 2015 Jul 10;6(19):17832-46. doi: 10.18632/oncotarget.4026. Oncotarget. 2015. PMID: 26098777 Free PMC article.
-
Identification of NHLRC1 as a Novel AKT Activator from a Lung Cancer Epigenome-Wide Association Study (EWAS).Int J Mol Sci. 2022 Sep 14;23(18):10699. doi: 10.3390/ijms231810699. Int J Mol Sci. 2022. PMID: 36142605 Free PMC article.
-
miR-708-5p: a microRNA with emerging roles in cancer.Oncotarget. 2017 Aug 1;8(41):71292-71316. doi: 10.18632/oncotarget.19772. eCollection 2017 Sep 19. Oncotarget. 2017. PMID: 29050362 Free PMC article. Review.
-
Nuclear FOXO3 predicts adverse clinical outcome and promotes tumor angiogenesis in neuroblastoma.Oncotarget. 2016 Nov 22;7(47):77591-77606. doi: 10.18632/oncotarget.12728. Oncotarget. 2016. PMID: 27769056 Free PMC article.
-
High-Level Expression, Purification and Large-Scale Production of l-Methionine γ-Lyase from Idiomarina as a Novel Anti-Leukemic Drug.Mar Drugs. 2015 Aug 21;13(8):5492-507. doi: 10.3390/md13085492. Mar Drugs. 2015. PMID: 26308011 Free PMC article.
References
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. - PubMed
-
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, Wu H, Li L. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441(7092):518–522. - PubMed
-
- Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Experimental Gerontology. 2006;41(8):709–717. - PubMed
-
- Zhao WL. Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways. Leukemia. 2009;24(1):13–21. - PubMed
-
- Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, Cocco L. Phosphoinositide 3-kinase//Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20(6):911–928. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

