Aldosterone: a mediator of retinal ganglion cell death and the potential role in the pathogenesis in normal-tension glaucoma
- PMID: 23828574
- PMCID: PMC3730414
- DOI: 10.1038/cddis.2013.240
Aldosterone: a mediator of retinal ganglion cell death and the potential role in the pathogenesis in normal-tension glaucoma
Abstract
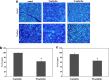
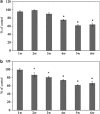
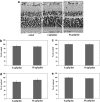
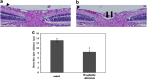
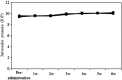
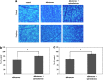
Glaucoma is conventionally defined as a chronic optic neuropathy characterized by progressive loss of retinal ganglion cells (RGCs) and optic nerve fibers. Although glaucoma is often associated with elevated intraocular pressure (IOP), significant IOP reduction does not prevent progression of the disease in some glaucoma patients. Thus, exploring IOP-independent mechanisms of RGC loss is important. We describe chronic systemic administration of aldosterone and evaluate its effect on RGCs in rat. Aldosterone was administered via an osmotic minipump that was implanted subcutaneously into the mid-scapular region. Although systemic administration of aldosterone caused RGC loss associated with thinning of the retinal nerve fiber layer without elevated IOP, the other cell layers appeared to be unaffected. After chronic administration of aldosterone, RGC loss was observed at 2 weeks in the peripheral retina and at 4 weeks in the central retina. However, administration of mineralocorticoid receptor blocker prevented RGC loss. These results demonstrate aldosterone is a critical mediator of RGC loss that is independent of IOP. We believe this rat normal-tension glaucoma (NTG) animal model not only offers a powerful system for investigating the mechanism of neurodegeneration in NTG, but can also be used to develop therapies directed at IOP-independent mechanisms of RGC loss.
Figures
Similar articles
-
The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma.J Clin Invest. 2007 Jul;117(7):1763-70. doi: 10.1172/JCI30178. J Clin Invest. 2007. PMID: 17607354 Free PMC article.
-
Upregulation of the endothelin A (ETA) receptor and its association with neurodegeneration in a rodent model of glaucoma.BMC Neurosci. 2017 Mar 1;18(1):27. doi: 10.1186/s12868-017-0346-3. BMC Neurosci. 2017. PMID: 28249604 Free PMC article.
-
Sirt6 protects retinal ganglion cells and optic nerve from degeneration during aging and glaucoma.Mol Ther. 2024 Jun 5;32(6):1760-1778. doi: 10.1016/j.ymthe.2024.04.030. Epub 2024 Apr 24. Mol Ther. 2024. PMID: 38659223 Free PMC article.
-
Regulated Cell Death of Retinal Ganglion Cells in Glaucoma: Molecular Insights and Therapeutic Potentials.Cell Mol Neurobiol. 2023 Oct;43(7):3161-3178. doi: 10.1007/s10571-023-01373-1. Epub 2023 Jun 20. Cell Mol Neurobiol. 2023. PMID: 37338781 Free PMC article. Review.
-
Glaucoma: the retina and beyond.Acta Neuropathol. 2016 Dec;132(6):807-826. doi: 10.1007/s00401-016-1609-2. Epub 2016 Aug 20. Acta Neuropathol. 2016. PMID: 27544758 Free PMC article. Review.
Cited by
-
Elevated plasma aldosterone levels are associated with a reduction in retinal ganglion cell survival.J Renin Angiotensin Aldosterone Syst. 2018 Jul-Sep;19(3):1470320318795001. doi: 10.1177/1470320318795001. J Renin Angiotensin Aldosterone Syst. 2018. PMID: 30129805 Free PMC article.
-
Is there any association between primary hyperparathyroidism and ocular changes, such as central corneal thickness, retinal thickness, and intraocular pressure?Endocrine. 2016 Mar;51(3):545-50. doi: 10.1007/s12020-015-0724-5. Epub 2015 Aug 29. Endocrine. 2016. PMID: 26318316
-
Genetic variation reveals the influence of steroid hormones on the risk of retinal neurodegenerative diseases.Front Endocrinol (Lausanne). 2023 Jan 10;13:1088557. doi: 10.3389/fendo.2022.1088557. eCollection 2022. Front Endocrinol (Lausanne). 2023. PMID: 36704044 Free PMC article.
-
Higher serum lipids and oxidative stress in patients with normal tension glaucoma, but not pseudoexfoliative glaucoma.Bosn J Basic Med Sci. 2016 Jan 1;16(1):21-7. doi: 10.17305/bjbms.2016.830. Bosn J Basic Med Sci. 2016. PMID: 26773174 Free PMC article.
-
Aldosterone as a mediator of severity in retinal vascular disease: Evidence and potential mechanisms.Exp Eye Res. 2019 Nov;188:107788. doi: 10.1016/j.exer.2019.107788. Epub 2019 Aug 31. Exp Eye Res. 2019. PMID: 31479654 Free PMC article. Review.
References
-
- Cartwright MJ, Anderson DR. Correlation of asymmetric damage with asymmetric intraocular pressure in normal-tension glaucoma (low-tension glaucoma) Arch Ophthalmol. 1988;106:898–900. - PubMed
-
- Crichton A, Drance SM, Douglas GR, Schulzer M. Unequal intraocular pressure and its relation to asymmetric visual field defects in low-tension glaucoma. Ophthalmology. 1989;96:1312–1314. - PubMed
-
- Abedin S, Simmons RJ, Grant WM. Progressive low-tension glaucoma: treatment to stop glaucomatous cupping and field loss when these progress despite normal intraocular pressure. Ophthalmology. 1982;89:1–6. - PubMed
-
- de Jong N, Greve EL, Hoyng PF, Geijssen HC. Results of a filtering procedure in low tension glaucoma. Int Ophthalmol. 1989;13:131–138. - PubMed
-
- Tezel G, Siegmund KD, Trinkaus K, Wax MB, Kass MA, Kolker AE. Clinical factors associated with progression of glaucomatous optic disc damage in treated patients. Arch Ophthalmol. 2001;119:813–818. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

