Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB
- PMID: 23828580
- PMCID: PMC6532682
- DOI: 10.1002/14651858.CD007545.pub2
Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB
Abstract
Background: Preventing active tuberculosis (TB) from developing in people with latent tuberculosis infection (LTBI) is important for global TB control. Isoniazid (INH) for six to nine months has 60% to 90% protective efficacy, but the treatment period is long, liver toxicity is a problem, and completion rates outside trials are only around 50%. Rifampicin or rifamycin-combination treatments are shorter and may result in higher completion rates.
Objectives: To compare the effects of rifampicin monotherapy or rifamycin-combination therapy versus INH monotherapy for preventing active TB in HIV-negative people at risk of developing active TB.
Search methods: We searched the Cochrane Infectious Disease Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; LILACS; clinical trials registries; regional databases; conference proceedings; and references, without language restrictions to December 2012; and contacted experts for relevant published, unpublished and ongoing trials.
Selection criteria: Randomized controlled trials (RCTs) of HIV-negative adults and children at risk of active TB treated with rifampicin, or rifamycin-combination therapy with or without INH (any dose or duration), compared with INH for six to nine months.
Data collection and analysis: At least two authors independently screened and selected trials, assessed risk of bias, and extracted data. We sought clarifications from trial authors. We pooled relative risks (RRs) with their 95% confidence intervals (CIs), using a random-effects model if heterogeneity was significant. We assessed overall evidence quality using the GRADE approach.
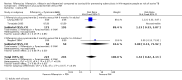
Main results: Ten trials are included, enrolling 10,717 adults and children, mostly HIV-negative (2% HIV-positive), with a follow-up period ranging from two to five years. Rifampicin (three/four months) vs. INH (six months)Five trials published between 1992 to 2012 compared these regimens, and one small 1992 trial in adults with silicosis did not detect a difference in the occurrence of TB over five years of follow up (one trial, 312 participants; very low quality evidence). However, more people in these trials completed the shorter course (RR 1.19, 95% CI 1.01 to 1.30; five trials, 1768 participants; moderate quality evidence). Treatment-limiting adverse events were not significantly different (four trials, 1674 participants; very low quality evidence), but rifampicin caused less hepatotoxicity (RR 0.12, 95% CI 0.05 to 0.30; four trials, 1674 participants; moderate quality evidence). Rifampicin plus INH (three months) vs. INH (six months)The 1992 silicosis trial did not detect a difference between people receiving rifampicin plus INH compared to INH alone for occurrence of active TB (one trial, 328 participants; very low quality evidence). Adherence was similar in this and a 1998 trial in people without silicosis (two trials, 524 participants; high quality evidence). No difference was detected for treatment-limiting adverse events (two trials, 536 participants; low quality evidence), or hepatotoxicity (two trials, 536 participants; low quality evidence). Rifampicin plus pyrazinamide (two months) vs. INH (six months)Three small trials published in 1994, 2003, and 2005 compared these two regimens, and two reported a low occurrence of active TB, with no statistically significant differences between treatment regimens (two trials, 176 participants; very low quality evidence) though, apart from one child from the 1994 trial, these data on active TB were from the 2003 trial in adults with silicosis. Adherence with both regimens was low with no statistically significant differences (four trials, 700 participants; very low quality evidence). However, people receiving rifampicin plus pyrazinamide had more treatment-limiting adverse events (RR 3.61, 95% CI 1.82 to 7.19; two trials, 368 participants; high quality evidence), and hepatotoxicity (RR 4.59, 95% 2.14 to 9.85; three trials, 540 participants; moderate quality evidence). Weekly, directly-observed rifapentine plus INH (three months) vs. daily, self-administered INH (nine months)A large trial conducted from 2001 to 2008 among close contacts of TB in the USA, Canada, Brazil and Spain found directly observed weekly treatment to be non-inferior to nine months self-administered INH for the incidence of active TB (0.2% vs 0.4%, RR 0.44, 95% CI 0.18 to 1.07, one trial, 7731 participants; moderate quality evidence). The directly-observed, shorter regimen had higher treatment completion (82% vs 69%, RR 1.19, 95% CI 1.16 to 1.22, moderate quality evidence), and less hepatotoxicity (0.4% versus 2.4%; RR 0.16, 95% CI 0.10 to 0.27; high quality evidence), though treatment-limiting adverse events were more frequent (4.9% versus 3.7%; RR 1.32, 95% CI 1.07 to 1.64 moderate quality evidence)
Authors' conclusions: Trials to date of shortened prophylactic regimens using rifampicin alone have not demonstrated higher rates of active TB when compared to longer regimens with INH. Treatment completion is probably higher and adverse events may be fewer with shorter rifampicin regimens. Shortened regimens of rifampicin with INH may offer no advantage over longer INH regimens. Rifampicin combined with pyrazinamide is associated with more adverse events. A weekly regimen of rifapentine plus INH has higher completion rates, and less liver toxicity, though treatment discontinuation due to adverse events is probably more likely than with INH.
Conflict of interest statement
None of the authors declare financial or academic conflicts of interest.
Figures










































Republished in
-
Rifamycins (rifampicin, rifabutin and rifapentine) compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB.Evid Based Child Health. 2014 Mar;9(1):169-294. doi: 10.1002/ebch.1962. Evid Based Child Health. 2014. PMID: 25404581
Comment in
-
Cochrane in context: Rifamycins compared to isoniazid for preventing tuberculosis in HIV-negative people at risk of active TB.Evid Based Child Health. 2014 Mar;9(1):295-7. doi: 10.1002/ebch.1959. Evid Based Child Health. 2014. PMID: 25404582 No abstract available.
References
References to studies included in this review
Chan 2012 {published and unpublished data}
-
- Chan PC, Yang CH, Chang LY, Wang KF, Lu BY, Lu CY, et al. Latent tuberculosis infection treatment for prison inmates: a randomised controlled trial. International Journal of Tuberculosis and Lung Diseases 2012;16(5):633‐8. - PubMed
HKCS 1992 {published data only}
-
- Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. A double‐blind placebo‐controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. The American Review of Respiratory Disease 1992;145(1):36‐41. - PubMed
Leung 2003 {published and unpublished data}
-
- Leung CC, Law WS, Chang KC, Tam CM, Yew WW, Chan CK, et al. Initial experience on rifampin and pyrazinamide versus isoniazid in the treatment of latent tuberculosis infection among patients with silicosis in Hong Kong. Chest 2003;124(6):2112‐8. - PubMed
Magdorf 1994 {published data only}
-
- Magdorf K, Arizzi‐Rusche AF, Geiter LJ, O'Brien RJ, Wahn U. [Compliance and tolerance of new antitubercular short‐term chemopreventive regimens in childhood‐‐a pilot project] [Compliance und Toleranz neuer antituberkulotischer Kurzzeit‐Chemopraventionsregime im Kindesalter‐‐eine Pilotstudie.]. Pneumologie (Stuttgart, Germany) 1994;48(10):761‐4. - PubMed
Martinez Alfaro 1998 {published data only}
-
- Martinez Alfaro E, Solera J, Serna E, Cuenca D, Castillejos ML, Espinosa A, et al. [Compliance, tolerance and effectiveness of a short chemoprophylaxis regimen for the treatment of tuberculosis] [Cumplimentacion, tolerancia y eficacia de una pauta corta de quimioprofilaxis para el tratamiento de la tuberculosis.]. Medicina clinica 1998;111(11):401‐4. - PubMed
Menzies 2004 {published data only}
-
- Menzies D, Dion MJ, Rabinovitch B, Mannix S, Brassard P, Schwartzman K. Treatment completion and costs of a randomized trial of rifampin for 4 months versus isoniazid for 9 months. American Journal of Respiratory and Critical Care Medicine 2004;170(4):445‐9. - PubMed
Menzies 2008 {published data only}
-
- Esfahani K, Aspler A, Menzies D, Schwartzman K. Rifampin versus isoniazid for treatment of latent tuberculosis: A cost‐effectiveness analysis based on a multicenter clinical trial. American Journal of Respiratory and Critical Care Medicine 2009;179:A101.
-
- Menzies D, Long R, Trajman A, Dion MJ, Yang J, Al Jahdali H, et al. Adverse events with four months of rifampin therapy or nine months of isoniazid therapy for latent tuberculosis infection: a randomized trial. Annals of Internal Medicine 2008;149(10):689‐97. - PubMed
-
- Trajman A, Zylberberg D, Dion MJ, Al‐Otaibi B, Long R, Oliveira AA, et al. Poster 46: 91161 Factors associated with treatment completion in a randomized trial of treatment of latent TB infection. Programme of the 5th Conference of The Union Europe Region, Dubrovnik, Croatia, 27‐30 May 2009; http://www.theunion.org/index.php/conferences/index.php?option=com_flexi.... (accessed 01 March 2012):131.
Sanchez‐Arcilla 2004 {published data only}
-
- Sanchez‐Arcilla I, Vilchez JM, Garcia de la Torre M, Fernandez X, Noguerado A. [Treatment of latent tuberculosis among homeless population. Comparison between two therapeutic approaches] [Infeccion tuberculosa latente en poblacion indigente. Comparacion de dos pautas terapeuticas.]. Medicina clinica 2004;122(2):57‐9. - PubMed
Sterling 2011 {published and unpublished data}
-
- Bliven‐Sizemore E, Sterling TR, Shang N, Benator D, Schwartzman K, Reves R, et al. Hepatitis C virus infection and female sex are risk factors for treatment limiting hepatotoxicity in a large clinical trial of treatment of latent tuberculosis infection: results of a nested case‐control study. American Journal of Respiratory and Critical Care Medicine 2011;183:A4041.
-
- CDC fact sheet. PREVENT TB study. http://www.cdc.gov/nchhstp/newsroom/docs/PREVENT‐TB‐Factsheet.pdf 2011; Vol. May:1‐2 (accessed 01 March 2012).
-
- Sterling T, Villarino E. TBTC study 26: weekly Rifapentine + INH for 3 months vs. daily INH for 9 months for the treatment of LTBI. http://clinicaltrials.gov/show/NCT00023452 issue accessed 14 November 2011.
-
- Sterling TR, Chaisson RE, Eng C, Hamilton CD, Gordin F, Hakman J, et al. A study of the effectiveness and tolerability of weekly Rifapentine/Isoniazid for three months versus daily Isoniazid for nine months for the treatment of latent tuberculosis infection, Version 12_19_06 (Study Protocol: US Public Health Service Study 26). New England Journal of Medicine 2011;365(23):1‐156.
-
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven‐Sizemore E, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. New England Journal of Medicine 2011;365(23):2155‐66. - PubMed
Tortajada 2005 {published and unpublished data}
-
- Tortajada C, Martinez‐Lacasa J, Sanchez F, Jimenez‐Fuentes A, Souza ML, Garcia JF, et al. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficency virus? Erratum. International Journal of Tuberculosis and Lung Diseases 2005;9(6):706. - PubMed
-
- Tortajada C, Martinez‐Lacasa J, Sanchez F, Jimenez‐Fuentes A, Souza ML, Garcia JF, et al. Is the combination of pyrazinamide plus rifampicin safe for treating latent tuberculosis infection in persons not infected by the human immunodeficiency virus?. The International Journal of Tuberculosis and Lung Disease 2005;9(3):276‐81. - PubMed
References to studies excluded from this review
Bailey 1974 {published data only}
-
- Bailey WC, Weill H, DeRouen TA, Ziskind MM, Jackson HA. The effect of isoniazid on transaminase levels. Annals of Internal Medicine 1974;81(2):200‐2. - PubMed
Barnwell 1992 {published data only}
Batki 2002 {published data only}
-
- Batki SL, Gruber VA, Bradley JM, Bradley M, Delucchi K. A controlled trial of methadone treatment combined with directly observed isoniazid for tuberculosis prevention in injection drug users. Drug and Alcohol Dependence 2002;66(3):283‐93. - PubMed
Bridge 1967 {published data only}
-
- Bridge EV. Chemoprophylaxis: a major adjunct in the prevention of tuberculosis. Michigan Medicine 1967;66(24):1553‐5. - PubMed
Byrd 1977 {published data only}
-
- Byrd RB, Horn BR, Griggs GA, Solomon DA. Isoniazid chemoprophylaxis. Association with detection and incidence of liver toxicity. Archives of internal medicine 1977;137(9):1130‐3. - PubMed
Catie 2001 {published data only}
-
- Infection fighters. Maintenance therapy for TB works. Treatment Update 2001; Vol. 12, issue 9:1‐2. - PubMed
Chaisson 2001 {published data only}
-
- Chaisson RE, Barnes GL, Hackman J, Watkinson L, Kimbrough L, Metha S, et al. A randomized, controlled trial of interventions to improve adherence to isoniazid therapy to prevent tuberculosis in injection drug users. The American Journal of Medicine 2001;110(8):610‐5. - PubMed
Coly 2004 {published data only}
-
- Coly A, Morisky D. Predicting completion of treatment among foreign‐born adolescents treated for latent tuberculosis infection in Los Angeles. The International Journal of Tuberculosis and Lung Disease 2004;8(6):703‐10. - PubMed
Comstock 1967 {published data only}
-
- Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community‐wide isoniazid prophylaxis in Alaska. The American Review of Respiratory Disease 1967;95(6):935‐43. - PubMed
Comstock 1972 {published data only}
-
- Comstock GW, Woolpert SF. Preventive treatment of untreated, nonactive tuberculosis in an Eskimo population. Archives of Environmental Health 1972;25(5):333‐7. - PubMed
Comstock 1974 {published data only}
-
- Comstock GW, Woolpert SF, Baum C. Isoniazid prophylaxis among Alaskan Eskimos: a progress report. The American Review of Respiratory Disease 1974;110(2):195‐7. - PubMed
Cowie 1996 {published data only}
-
- Cowie RL. Short course chemoprophylaxis with rifampicin, isoniazid and pyrazinamide for tuberculosis evaluated in gold miners with chronic silicosis: a double‐blind placebo controlled trial. Tuberclosis and Lung Disease 1996;77(3):239‐43. - PubMed
Debre 1973 {published data only}
-
- Debre R, Perdrizet S, Lotte A, Naveau M, Lert F. Isoniazid chemoprophylaxis of latent primary tuberculosis: in five trial centres in France from 1959 to 1969. International Journal of Epidemiology 1973;2(2):153‐60. - PubMed
Egsmose 1965 {published data only}
Eule 1973 {published data only}
-
- Eule H, Lucchesi M, Ozgen ZS, Zierski M. [Double blind study of the toxicity of different doses of ethambutol given in an intermittent therapeutic regimen with streptomycin, isoniazide and ethambutol] [Etude double aveugle sur la toxicite de differentes doses d'ethambutol donnees dans un regime therapeutique intermittent comprenant streptomycine, isoniazide et ethambutol]. Bulletin of the International Union against Tuberculosis 1973;48:112‐6. - PubMed
Eule 1973a {published data only}
-
- Eule H, Karnbach E, Kaluza P, Herrmann H. Preliminary results of a controlled therapeutic trail administering INH‐RMP once‐weekly, after‐ or without‐ an initial period of continuous treatment. Scandinavian Journal of Respiratory Diseases. Supplementum 1973;84:153‐9. - PubMed
Felten 1989 {published data only}
-
- Felten MK, Merwe CA. Random variation in tuberculin sensitivity in schoolchildren. Serial skin testing before and after preventive treatment for tuberculosis. The American Review of Respiratory Disease 1989;140(4):1001‐6. [PUBMED: 2802363] - PubMed
Ferebee 1962 {published data only}
-
- Ferebee SH, Mount FW. Tuberculosis morbidity in a controlled trial of the prophylactic use of isoniazid among household contacts. The American Review of Respiratory Disease 1962;85:490‐510. - PubMed
Ferebee 1963 {published data only}
-
- Ferebee SH, Mount FW, Murray FJ, Livesay VT. A controlled trial of isoniazid prophylaxis in mental institutions. The American Review of Respiratory Disease 1963;88:161‐75. - PubMed
Ferebee 1968 {published data only}
-
- Ferebee SH. Long‐term effects of isoniazid prophylaxis. Bulletin of the International Union against Tuberculosis 1968;41:161‐6. - PubMed
Fielding 2011 {published data only}
-
- Fielding KL, Grant AD, Hayes RJ, Chaisson RE, Corbett EL, Churchyard GJ. Thibela TB: design and methods of a cluster randomised trial of the effect of community‐wide isoniazid preventive therapy on tuberculosis amongst gold miners in South Africa. Contemporary Clinical Trials 2011;32(3):382‐92. - PubMed
Frigati 2011 {published data only}
-
- Frigati LJ, Kranzer K, Cotton MF, Schaaf HS, Lombard CJ, Zar HJ. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax 2011;66(6):496‐501. - PubMed
Gao 2006 {published data only}
-
- Gao XF, Wang L, Liu GJ, Wen J, Sun X, Xie Y, et al. Rifampicin plus pyrazinamide versus isoniazid for treating latent tuberculosis infection: a meta‐analysis. The International Journal of Tuberculosis and Lung disease 2006;10(10):1080‐90. - PubMed
Geijo 2007 {published data only}
-
- Geijo MP, Herranz CR, Vano D, Garcia AJ, Garcia M, Dimas JF. [Short‐course isoniazid and rifampin compared with isoniazid for latent tuberculosis infection: a randomized clinical trial] [Pauta corta de isoniazida y rifampicina comparada con isoniazida para la infeccion latente de tuberculosis. Ensayo clinico aleatorizado.]. Enfermedades infecciosas y microbiologia clinica 2007;25(5):300‐4. - PubMed
Geiter 1987 {published data only}
-
- Geiter LJ, O'Brien RJ, Combs DL, Snider DE Jr. United States Public Health Service Tuberculosis Therapy Trial 21: preliminary results of an evaluation of a combination tablet of isoniazid, rifampin and pyrazinamide. Tubercle 1987;68(2 Suppl):41‐6. - PubMed
Glassroth 1977 {published data only}
-
- Glassroth JL, White MC, Snider DE Jr. An assessment of the possible association of isoniazid with human cancer deaths. The American Review of Respiratory Disease 1977;116(6):1065‐74. - PubMed
Graham 1996 {published data only}
-
- Graham NM, Galai N, Nelson KE, Astemborski J, Bonds M, Rizzo RT, et al. Effect of isoniazid chemoprophylaxis on HIV‐related mycobacterial disease. Archives of Internal Medicine 1996;156(8):889‐94. - PubMed
Gupta 1993 {published data only}
-
- Gupta DK, Kumar R, Nath N, Kothari AK. Chemoprophylaxis in high risk children‐analysis of eight years' follow‐ up: Preliminary report. The Indian Journal of Tuberculosis 1993;40:125‐7.
Horwitz 1966 {published data only}
Horwitz 1974 {published data only}
-
- Horwitz O, Magnus K. Epidemiologic evaluation of chemoprophylaxis against tuberculosis. American Journal of Epidemiology 1974;99(5):333‐42. - PubMed
IUAT 1982 {published data only}
Jasmer 2002b {published data only}
-
- Jasmer R, Bernardo J, Daley C, Merrifield C, Blumberg H, Saukkonen J, et al. Short course rifampin and pyrazinamide versus isoniazid for latent tuberculosis infection: a multicentre prospective, randomized controlled trial [abstract]. American Journal of Respiratory and Critical Care Medicine. 2000; Vol. 161:A524.
-
- Jasmer RM, Saukkonen JJ, Blumberg HM, Daley CL, Bernardo J, Vittinghoff E, et al. Short‐course rifampin and pyrazinamide compared with isoniazid for latent tuberculosis infection: a multicenter clinical trial. Annals of Internal Medicine 2002;137(8):640‐7. - PubMed
John 1994 {published data only}
-
- John GT, Thomas PP, Jacob CK, Thomas M, Kirubakaran MG, Shastry JCM. Double blind randomised trial of primary INAH prophylaxis in dialysis and transplant patients. 12th International Congress of Nephrology. Israel. 2000; Vol. 166:13‐18.
-
- John GT, Thomas PP, Thomas M, Jeyaseelan L, Jacob CK, Shastry JC. A double‐blind randomized controlled trial of primary isoniazid prophylaxis in dialysis and transplant patients. Transplantation 1994;57(11):1683‐4. [PUBMED: 8009608] - PubMed
Krebs 1977 {published data only}
-
- Krebs A. [The role of preventive chemotherapy in the present tuberculosis epidemiology of GDR (author's translation)] [Die Bedeutung der praventiven Chemotherapie in der gegenwartigen Tuberkulosesituation der DDR]. Zeitschrift fur Erkrankungen der Atmungsorgane 1977;147(1):18‐25. - PubMed
Krebs 1979 {published data only}
-
- Krebs A, Farer LS, Snider WE, Thompson NJ. Five years of follow‐up of the IUAT trial of isoniazid prophylaxis in fibrotic lesions. Bulletin of the International Union against Tuberculosis 1979;54:65.
Krebs 1980 {published data only}
-
- Krebs A, Claiciu I. Late results of preventive chemotherapy in persons with fibrotic lesions of the lung; benefit, cost and risk (from the IUAT isoniazid prophylaxis trial). Revista de igiena, bacteriologie, virusologie, parazitologie, epidemiologie, pneumoftiziologie. Pneumoftiziologia 1980;29(2):77‐82. - PubMed
Lienhardt 2011 {published data only}
-
- Lienhardt C, Cook SV, Burgos M, Yorke‐Edwards V, Rigouts L, Anyo G, et al. Efficacy and safety of a 4‐drug fixed‐dose combination regimen compared with separate drugs for treatment of pulmonary tuberculosis: the Study C randomized controlled trial. Journal of the American Medical Association 2011;305(14):1415‐23. - PubMed
Madhi 2011 {published data only}
Martinson 2011 {published data only}
Moulton 2007 {published data only}
-
- Moulton LH, Golub JE, Durovni B, Cavalcante SC, Pacheco AG, Saraceni V, et al. Statistical design of THRio: a phased implementation clinic‐randomized study of a tuberculosis preventive therapy intervention. Clinical Trials (London, England) 2007;4(2):190‐9. - PubMed
Mount 1962 {published data only}
-
- Mount FW, Ferebee SH. The effect of isoniazid prophylaxis on tuberculosis morbidity among household contacts of previously known cases of tuberculosis. The American Review of Respiratory Disease 1962;85:821‐7. [PUBMED: 14476668] - PubMed
Nazareth 1971 {published data only}
-
- Nazareth O, Devadatta S, Fox W, Menon NK, Radhakrishna S, Rajappa D, et al. Two controlled studies of the efficacy of isoniazid alone in preventing relapse in patients with bacteriologically quiescent pulmonary tuberculosis at the end of one year of chemotherapy. Bulletin of the World Health Organization 1971;45(5):603‐15. [PUBMED: 4947494] - PMC - PubMed
Nunn 2011 {published data only}
-
- Nunn AJ, Jindani A, Enarson DA. Results at 30 months of a randomised trial of two eight‐month regimens for the treatment of tuberculosis. International Journal of Tuberculosis and Lung Diseases 2011;15(6):741‐45. - PubMed
Samandari 2011 {published data only}
-
- Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, et al. 6‐month versus 36‐month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double‐blind, placebo‐controlled trial. Lancet 2011;377(9777):1588‐98. - PubMed
Schechter 2006 {published data only}
Spyridis 2007 {published data only}
-
- Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, et al. The effectiveness of a 9‐month regimen of isoniazid alone versus 3‐ and 4‐month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11‐year randomized study. Clinical Infectious Diseases 2007;45(6):715‐22. - PubMed
Veening 1968 {published data only}
-
- Veening GJ. Long term isoniazid prophylaxis. Controlled trial on INH prophylaxis after recent tuberculin conversion in young adults. Bulletin of the International Union against Tuberculosis 1968;41:169‐71. - PubMed
References to studies awaiting assessment
White 2012 {published and unpublished data}
-
- NCT00128206. Treatment of Latent TB Infection for Jailed Persons. http://clinicaltrials.gov/ct2/show/NCT00128206 (Accessed 10 May 2012).
References to ongoing studies
ISRCTN53253537 {published data only}
-
- Menzies D. A randomized trial to compare completion and tolerability of 4 months rifampin (4 Rif) and 9 months isoniazid (9 INH) in treatment of latent TB in children. http://www.controlled‐trials.com/ISRCTN53253537 Accessed 14 November 2010.
NCT00023452 {published data only}
-
- CDC fact sheet. PREVENT TB study. http://www.cdc.gov/nchhstp/newsroom/docs/PREVENT‐TB‐Factsheet.pdf 2011; Vol. May:1‐2 (Accessed 14 November 2011).
-
- Sterling T, Villarino E. TBTC study 26: weekly Rifapentine + INH for 3 months versus daily INH for 9 months for the treatment of LTBI. http://clinicaltrials.gov/show/NCT00023452 (Accessed 14 November 2011).
NCT00931736 {published data only}
-
- Menzies D. A randomized clinical trial comparing 4RIF versus 9INH for treatment of latent tuberculosis infection (LTBI) ‐ effectiveness. http://clinicaltrials.gov/show/NCT00931736 (Accessed 14 November 2011).
NCT01398618 {published data only}
-
- Wang JY. Comparing the efficacy of two preventive regimens for adult household contacts with latent tuberculosis infection. http://clinicaltrials.gov/ct2/show/record/NCT01398618 (Accessed 15 April 2012).
Additional references
Ahmad 2011
Akolo 2010
Andrade 2011
Aspler 2010
-
- Aspler A, Long R, Trajman A, Dion MJ, Khan K, Schwartzman K, et al. Impact of treatment completion, intolerance and adverse events on health system costs in a randomised trial of 4 months rifampin or 9 months isoniazid for latent TB. Thorax 2010;65:582‐87. - PubMed
ATS 1990
-
- American Thoracic Society. Diagnostic standards and classification of tuberculosis. American Review of Respiratory Disease 1990;142(3):725‐35. - PubMed
ATS/CDC 2003
-
- ATS/CDC. Update: adverse event data and revised American ThoracicSociety/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection‐ United States, 2003. Morbidity and Mortality Weekly Report 2003;52(31):735‐9. - PubMed
Balcells 2006
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. - PubMed
Barboza 2008
-
- Barboza CE, Winter DH, Seiscento M, Santos UP, Terra Filho M. Tuberculosis and silicosis: epidemiology, diagnosis and chemoprophylaxis [Tuberculose e silicose: epidemiologia, diagnóstico e quimioprofilaxia]. Jornal Brasileiro de Pneumologia 2008;34(11):959‐66. - PubMed
Barry 2009
Baussano 2011
Benator 2002
-
- Benator D, Bhattacharya M, Bozeman L, Burman W, Cantazaro A, Chaisson R, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug‐susceptible pulmonary tuberculosis in HIV‐negative patients: a randomised clinical trial. Lancet 2002;360(9332):528‐34. - PubMed
Blumberg 2003
-
- Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. American Journal of Respiratory and Critical Care Medicine 2003;167(4):603‐62. - PubMed
Bock 2002
-
- Bock NN, Sterling TR, Hamilton CD, Pachucki C, Wang YC, Conwell DS, et al. A prospective, randomized, double‐blind study of the tolerability of rifapentine 600, 900, and 1,200 mg plus isoniazid in the continuation phase of tuberculosis treatment. American Journal of Respiratory and Critical Care Medicine 2002;165(11):1526‐30. - PubMed
Cattamanchi 2011
-
- Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, et al. Interferon‐gamma release assays for the diagnosis of latent tuberculosis infection in HIV‐infected individuals: a systematic review and meta‐analysis. Journal of Acquired Immune Deficiency Syndrome 2011;56(3):230‐8. - PMC - PubMed
CDC 2000
-
- Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. Montly Morbidity and Mortality Weekly Report 2000;49:1‐51. - PubMed
CDC 2002
-
- Centers for Disease Control and Prevention. Acquired rifamycin resistance in persons with advanced HIV disease being treated for active tuberculosis with intermittent rifamycin‐based regimens. Morbidity and Mortality Weekly Report 2002;51(10):214‐5. - PubMed
CDC 2010
-
- Centers for Disease Control and Prevention. Severe isoniazid‐associated liver injuries among persons being treated for latent tuberculosis infection‐United States, 2004–2008. Morbidity and Mortality Weekly Report 2010;59(8):224‐9. - PubMed
CDC 2011
-
- Center for Disease Control and Prevention. Recommendations for use of an isoniazid‐rifapentine regimen with direct observation to treat latent Mycobacterium tuberculosis infection. Morbidity and Mortality Weekly Report 2011;60(48):1650‐53. - PubMed
Christopher 2011
Cook 2006
-
- Cook PP, Maldonado RA, Yarnell CT, Holbert D. Safety and completion rate of short‐course therapy for treatment of latent tuberculosis infection. Clinical Infectious Diseases 2006;43(3):271‐5. - PubMed
Corbiere 2012
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Diel 2011
-
- Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, et al. Interferon‐γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta‐analysis. European Respiratory Journal 2011;37(1):88‐99. - PubMed
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. - PubMed
Dyrhol‐Riise, 2010
-
- Dyrhol‐Riise AM, Gran G, Wentzel‐Larsen T, Blomberg B, Haanshuus CG, Morkve O. Diagnosis and follow‐up of treatment of latent tuberculosis; the utility of the QuantiFERON‐TB Gold In‐tube assay in outpatients from a tuberculosis low‐endemic country . BMC Infectious Diseases 2010;10:57. - PMC - PubMed
Ena 2005
-
- Ena J, Valls V. Short‐course therapy with rifampin plus isoniazid compared with standard therapy with isoniazid for latent tuberculosis Infection: a meta‐analysis. Clinical Infectious Diseases 2005;40(5):670‐6. - PubMed
Esfahani 2009
-
- Esfahani K, Aspler A, Menzies D, Schwartzman K. Rifampin versus isoniazid for treatment of latent tuberculosis: A cost‐effectiveness analysis based on a multicenter clinical trial. American Journal of Respiratory and Critical Care Medicine 2009;179:A101.
Frieden 2003
-
- Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet 2003;362(9387):887‐99. - PubMed
Gordin 2004
-
- Gordin FM, Cohn DL, Matts JP, Chaisson RE, O'Brien RJ. Hepatotoxicity of rifampin and pyrazinamide in the treatment of latent tuberculosis infection in HIV‐infected persons: is it different than in HIV‐uninfected persons?. Clinical Infectious Diseases 2004;39(4):561‐5. - PubMed
GRADE 2004 [Computer program]
-
- GRADE Working Group. GRADE Profiler; version 3.6. GRADE Working Group, 2004.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration..
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Holland 2009
Holland 2011
Horsburgh 2009
-
- Horsburgh CR, Jr, Goldberg S, Bethel J, Chen S, Colson PW, Hirsch‐Moverman Y, et al. Latent TB infection treatment acceptance and completion in the United States and Canada. Chest 2009;137(2):401‐9. - PubMed
Houben 2011
-
- Houben RM, Crampin AC, Ndhlovu R, Sonnenberg P, Godfrey‐Faussett P, Haas WH, et al. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. International Journal of Tuberculosis and Lung Diseases 2011;15(1):24‐31. - PubMed
Jasmer 2002a
-
- Jasmer RM, Nahid P, Hopewell PC. Latent tuberculosis infection. New England Journal of Medicine 2002;347(23):1860‐6. - PubMed
Joshi 2006
Khan 2002
-
- Khan K, Muennig P, Behta M, Zivin JG. Global drug‐resistance patterns and the management of latent tuberculosis infection in immigrants to the United States. New England Journal of Medicine 2002;347(23):1850‐9. - PubMed
Lardizabal 2006
-
- Lardizabal A, Passannante M, Kojakali F, Hayden C, Reichman LB. Enhancement of treatment completion for latent tuberculosis infection with 4 months of rifampin. Chest 2006;130(6):1712‐7. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lin 2010
LoBue 2003
-
- LoBue PA, Moser KS. Use of isoniazid for latent tuberculosis infection in a public health clinic. American Journal of Respiratory and Critical Care Medicine 2003;168(4):443‐7. - PubMed
Lobue 2010
-
- LoBue P, Menzies D. Treatment of latent tuberculosis infection: an update. Respirology 2010;15:603‐22. - PubMed
Ma 2011
Machingaidze 2011
-
- Machingaidze S, Wiysonge CS, Gonzalez‐Angulo Y, Hatherill M, Moyo S, Hanekom W, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta‐analysis. Pediatric Infectious Disease Journal 2011;30(8):694‐700. - PubMed
Marais 2006
McElroy 2005
-
- McElroy PD, Ijaz K, Lambert LA, Jereb JA, Iademarco MF, Castro KG, et al. National survey to measure rates of liver injury, hospitalization, and death associated with rifampin and pyrazinamide for latent tuberculosis infection. Clinical Infectious Diseases 2005;41(8):1125‐33. - PubMed
MECIR 2011
-
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Methodological standards for the conduct of new Cochrane Intervention Reviews. Version 2.1, 8 December 2011. Available at: http://www.editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR_.... (accessed on 4 November 2012).
Nardell 2011
-
- Nardell E, Churchyard G. What is thwarting tuberculosis prevention in high‐burden settings?. New England Journal of Medicine 2011;365(1):79‐81. - PubMed
NCT01582711
-
- Centers for Disease Control and Prevention. TBTC Study 33. An evaluation of adherence to latent tuberculosis Infection (LTBI) treatment With 12 Doses of once weekly rifapentine (RPT) and isoniazid (INH) given as self‐administered (SAT) versus directly‐observed therapy (DOT): (iAdhere). Available at: http://www.clinicaltrials.gov/ct2/show/study/NCT01582711?term=rifapentin... 2012:(Accessed 8 May 2012).
NICE 2011
-
- National Institute for Health and Clinical Excellence, National Collaborating Centre for Chronic Conditions. 1.6 Treatment of latent TB infection. NICE clinical guideline 117: Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. London: National Institute for Health and Clinical Excellence, 2011:30‐34.
Noyes 2007
-
- Noyes J, Popay J. Directly observed therapy and tuberculosis: how can a systematic review of qualitative research contribute to improving services? A qualitative meta‐synthesis. Journal of Advanced Nursing 2007;57:227‐43. - PubMed
NYC 2005
-
- New York City Department of Health & Mental Hygiene, Bureau of Tuberculosis Control. Guidelines for testing and treatment of latent tuberculosis infection. Avialable at: www.nyc.gov/html/doh/downloads/pdf/tb/ltbi_guidelines.pdf. 2005:(Accessed 01 May 2012).
Pai 2005
-
- Pai M, Gokhale K, Joshi R, Dogra S, Kalantri S, Mendiratta DK, et al. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole‐blood interferon gamma assay with tuberculin skin testing. Journal of the American Medical Association 2005;293(22):2746‐55. - PubMed
Pai 2008
Pai 2011
-
- Pai M, Joshi R, Narang U, Zwerling A, Jain V, Kalantri S. Predictive value of IGRA and TST in Indian health‐care workers: A six‐year follow up study. American Journal of Respiratory and Critical Care Medicine. 2011; Vol. 183:A1198.
Rangaka 2012
Reichman 2004
-
- Reichman LB, Lardizabal A, Hayden CH. Considering the role of four months of rifampin in the treatment of latent tuberculosis infection. American Journal of Respiratory and Critical Care Medicine 2004;170(8):832‐5. - PubMed
Review Manager (RevMan) [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Ridzon 2005
-
- Ridzon R. Optimal latent TB control methods. International Journal of Tuberculosis and Lung Diseases 2005;9(3):236. - PubMed
Schunemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Hanbook for Systematic Review of Intervention Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Sester 2011
-
- Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, et al. Interferon‐γ release assays for the diagnosis of active tuberculosis: a systematic review and meta‐analysis. European Respiratory Journal 2011;37(1):100‐11. - PubMed
Smieja 1999
Stout 2010
-
- Stout JE, Holland DP. Treating latent tuberculosis with rifampin: is it the cheaper option?. Thorax 2010;65(7):572‐3. - PubMed
TBCTA 2009
-
- Tuberculosis Coalition for Technical Assistance and the International Committee of the Red Cross. Guidelines for control of Tuberculosis in Prisons. USAID/TBCTA, 2009.
van der Werf 2012
-
- Werf MJ, Langendam MW, Sandgren A, Manissero D. Lack of evidence to support policy development for management of contacts of multidrug‐resistant tuberculosis patients: two systematic reviews. International Journal of Tuberculosis and Lung Disease 2012;16(3):288‐96. - PubMed
van Zyl 2006
-
- Zyl S, Marais BJ, Hesseling AC, Gie RP, Beyers N, Schaaf HS. Adherence to anti‐tuberculosis chemoprophylaxis and treatment in children. International Journal of Tuberculosis and Lung Disease 2006;10(1):13‐8. - PubMed
Volmink 2007
WHO 2004
-
- World Health Organization. International Union against Tuberculosisand Lung Disease. Anti‐tuberculosis drug resistance in the world, Report no. 3. Geneva: WHO Stop TB, 2004.
WHO 2007
-
- World Health Organization Stop TB Partnership Childhood TB Subgroup. Chapter 4: Childhood contact screening and management. International Journal of Tuberculosis and Lung Disease 2007;11(1):12‐5. - PubMed
WHO 2008
-
- World Health Organization. Guidelines for the programmatic management of drug‐resistant tuberculosis. Emergency Update 2008. Geneva: World Health Organization, 2008.
WHO 2010a
-
- World Health Organization. Key points. Global tuberculosis control: surveillance, planning, financing; WHO report 2010 [WHO/HTM/TB/2010.7]. Geneva: World Health Organization, 2010:1‐6.
WHO 2010b
-
- World Health Organization. Treatment of tuberculosis. Guidelines. 4th Edition. Geneva: World Health Organization, 2010.
WHO 2011a
-
- World Health Organization. WHO Report 2011: Global Tuberculosis Control. Geneva: World Health Organization, 2011.
WHO 2011b
-
- World Health Organization. Guidelines for the programmatic management of drug‐resistant tuberculosis. 2011 update. Geneva: World Health Organization, 2011.
WHO 2011c
-
- World Health Organization. Guidelines for intensified tuberculosis case‐finding and isoniazid preventive therapy for people living with HIV in resource‐constrained settings. Geneva: World Health Organization, 2011.
WHO 2012
-
- World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization, 2012.
Zhang 2009
Ziakas 2009
-
- Ziakas PD, Mylonakis E. 4 months of rifampin compared with 9 months of isoniazid for the management of latent tuberculosis infection: a meta‐analysis and cost‐effectiveness study that focuses on compliance and liver toxicity. Clinical Infectious Diseases 2009;49(12):1883‐9. - PubMed
Zuniga 2012
Zwerling 2012
-
- Zwerling A, Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon‐gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 2012;67(1):62‐70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

