Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea
- PMID: 23828581
- PMCID: PMC6532719
- DOI: 10.1002/14651858.CD009029.pub2
Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea
Abstract
Background: Infection with enterotoxigenic Escherichia coli (ETEC) bacteria is a common cause of diarrhoea in adults and children in developing countries and is a major cause of 'travellers' diarrhoea' in people visiting or returning from endemic regions. A killed whole cell vaccine (Dukoral®), primarily designed and licensed to prevent cholera, has been recommended by some groups to prevent travellers' diarrhoea in people visiting endemic regions. This vaccine contains a recombinant B subunit of the cholera toxin that is antigenically similar to the heat labile toxin of ETEC. This review aims to evaluate the clinical efficacy of this vaccine and other vaccines designed specifically to protect people against diarrhoea caused by ETEC infection.
Objectives: To evaluate the efficacy, safety, and immunogenicity of vaccines for preventing ETEC diarrhoea.
Search methods: We searched the Cochrane Infectious Disease Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS, and http://clinicaltrials.gov up to December 2012.
Selection criteria: Randomized controlled trials (RCTs) and quasi-RCTs comparing use of vaccines to prevent ETEC with use of no intervention, a control vaccine (either an inert vaccine or a vaccine normally given to prevent an unrelated infection), an alternative ETEC vaccine, or a different dose or schedule of the same ETEC vaccine in healthy adults and children living in endemic regions, intending to travel to endemic regions, or volunteering to receive an artificial challenge of ETEC bacteria.
Data collection and analysis: Two authors independently assessed each trial for eligibility and risk of bias. Two independent reviewers extracted data from the included studies and analyzed the data using Review Manager (RevMan) software. We reported outcomes as risk ratios (RR) with 95% confidence intervals (CI). We assessed the quality of the evidence using the GRADE approach.
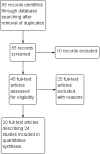
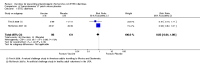
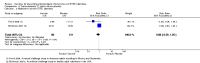
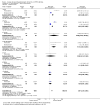
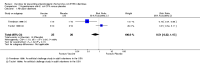
Main results: Twenty-four RCTs, including 53,247 participants, met the inclusion criteria. Four studies assessed the protective efficacy of oral cholera vaccines when used to prevent diarrhoea due to ETEC and seven studies assessed the protective efficacy of ETEC-specific vaccines. Of these 11 studies, seven studies presented efficacy data from field trials and four studies presented efficacy data from artificial challenge studies. An additional 13 trials contributed safety and immunological data only. Cholera vaccinesThe currently available, oral cholera killed whole cell vaccine (Dukoral®) was evaluated for protection of people against 'travellers' diarrhoea' in a single RCT in people arriving in Mexico from the USA. We did not identify any statistically significant effects on ETEC diarrhoea or all-cause diarrhoea (one trial, 502 participants, low quality evidence).Two earlier trials, one undertaken in an endemic population in Bangladesh and one undertaken in people travelling from Finland to Morocco, evaluated a precursor of this vaccine containing purified cholera toxin B subunit rather than the recombinant subunit in Dukoral®. Short term protective efficacy against ETEC diarrhoea was demonstrated, lasting for around three months (RR 0.43, 95% CI 0.26 to 0.71; two trials, 50,227 participants). This vaccine is no longer available. ETEC vaccinesAn ETEC-specific, killed whole cell vaccine, which also contains the recombinant cholera toxin B-subunit, was evaluated in people travelling from the USA to Mexico or Guatemala, and from Austria to Latin America, Africa, or Asia. We did not identify any statistically significant differences in ETEC-specific diarrhoea or all-cause diarrhoea (two trials, 799 participants), and the vaccine was associated with increased vomiting (RR 2.0, 95% CI 1.16 to 3.45; nine trials, 1528 participants). The other ETEC-specific vaccines in development have not yet demonstrated clinically important benefits.
Authors' conclusions: There is currently insufficient evidence from RCTs to support the use of the oral cholera vaccine Dukoral® for protecting travellers against ETEC diarrhoea. Further research is needed to develop safe and effective vaccines to provide both short and long-term protection against ETEC diarrhoea.
Conflict of interest statement
We certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of the review (eg employment, consultancy, stock ownership, honoraria, and expert testimony).
Figures

























Update of
- doi: 10.1002/14651858.CD009029
Similar articles
-
Oral killed cholera vaccines for preventing cholera.Cochrane Database Syst Rev. 2024 Jan 10;1(1):CD014573. doi: 10.1002/14651858.CD014573. Cochrane Database Syst Rev. 2024. PMID: 38197546 Free PMC article.
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2012 Nov 14;11:CD008521. doi: 10.1002/14651858.CD008521.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2019 Mar 25;3:CD008521. doi: 10.1002/14651858.CD008521.pub4. PMID: 23152260 Updated.
-
Oral vaccines for preventing cholera.Cochrane Database Syst Rev. 2011 Mar 16;2011(3):CD008603. doi: 10.1002/14651858.CD008603.pub2. Cochrane Database Syst Rev. 2011. PMID: 21412922 Free PMC article.
-
Vaccines for preventing typhoid fever.Cochrane Database Syst Rev. 2018 May 31;5(5):CD001261. doi: 10.1002/14651858.CD001261.pub4. Cochrane Database Syst Rev. 2018. PMID: 29851031 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
Cited by
-
Preclinical optimization of an enterotoxigenic Escherichia coli adjuvanted subunit vaccine using response surface design of experiments.NPJ Vaccines. 2020 Sep 11;5:83. doi: 10.1038/s41541-020-00228-w. eCollection 2020. NPJ Vaccines. 2020. PMID: 32983577 Free PMC article.
-
Use of Ensure® nutrition shakes as an alternative formulation method for live recombinant Attenuated Salmonella Typhi vaccines.BMC Microbiol. 2015 Mar 29;15:76. doi: 10.1186/s12866-015-0409-5. BMC Microbiol. 2015. PMID: 25879849 Free PMC article.
-
Small Intestinal Infections.Curr Gastroenterol Rep. 2016 Jun;18(6):31. doi: 10.1007/s11894-016-0502-4. Curr Gastroenterol Rep. 2016. PMID: 27168147 Review.
-
A Review of Guidelines/Guidance from Various Countries Around the World for the Prevention and Management of Travellers' Diarrhoea: A Pharmacist's Perspective.Pharmacy (Basel). 2019 Aug 4;7(3):107. doi: 10.3390/pharmacy7030107. Pharmacy (Basel). 2019. PMID: 31382691 Free PMC article. Review.
-
The Virulence Regulator Rns Activates the Expression of CS14 Pili.Genes (Basel). 2016 Dec 8;7(12):120. doi: 10.3390/genes7120120. Genes (Basel). 2016. PMID: 27941642 Free PMC article.
References
References to studies included in this review
Clemens 1988 {published data only}
-
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Neogy PK, Stanton B, et al. Cross‐protection by B subunit‐whole cell cholera vaccine against diarrhea associated with heat‐labile toxin‐producing enterotoxigenic Escherichia coli: results of a large‐scale field trial. Journal of Infectious Diseases 1988;158(2):372‐7. - PubMed
-
- Clemens JD, Stanton BF, Harris JR, Chakraborty J, Sack DA, Rao MR, et al. Exclusion of clinically atypical or microbiologically mixed diarrhoeal episodes from outcome events in a field trial of oral cholera vaccines. International Journal of Epidemiology 1989;18(2):440‐5. - PubMed
Cohen 2000 (Study 1) {published data only}
Cohen 2000 (Study 2) {published data only}
Frech 2008 {published data only}
-
- Frech SA, Dupont HL, Bourgeois AL, McKenzie R, Belkind‐Gerson J, Figueroa JF, et al. Use of a patch containing heat‐labile toxin from Escherichia coli against travellers' diarrhoea: a phase II, randomised, double‐blind, placebo‐controlled field trial. Lancet 2008;371(9629):2019‐25. - PubMed
Freedman 1998 {published data only}
-
- Freedman DJ, Tacket C O, Delehanty A, Maneval DR, Nataro J, Crabb JH. Milk immunoglobulin with specific activity against purified colonization factor antigens can protect against oral challenge with enterotoxigenic Escherichia coli. Journal of Infectious Diseases 1998;177(3):662‐7. - PubMed
Hall 2001 (Study 1) {published data only}
-
- Hall ER, Wierzba TF, Ahren C, Rao MR, Bassily S, Francis W, et al. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenicEscherichia coli plus cholera toxin B subunit vaccine. Infection & Immunity 2001;69(5):2853‐7. - PMC - PubMed
Hall 2001 (Study 2) {published data only}
-
- Hall ER, Wierzba TF, Ahren C, Rao MR, Bassily S, Francis W, et al. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenicEscherichia coli plus cholera toxin B subunit vaccine. Infection & Immunity 2001;69(5):2853‐7. - PMC - PubMed
Hall 2001 (Study 3) {published data only}
-
- Hall ER, Wierzba TF, Ahren C, Rao MR, Bassily S, Francis W, et al. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenicEscherichia coli plus cholera toxin B subunit vaccine. Infection & Immunity 2001;69(5):2853‐7. - PMC - PubMed
Jertborn 1998 {published data only}
-
- Jertborn M, Ahren C, Holmgren J, Svennerholm AM. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 1998;16(2‐3):255‐60. - PubMed
Jertborn 2001 {published data only}
Leyten 2005 {published data only}
-
- Leyten EM, Soonawala D, Schultsz C, Herzog C, Ligthelm RJ, Wijnands S, et al. Analysis of efficacy of CVD 103‐HgR live oral cholera vaccine against all‐cause travellers' diarrhoea in a randomised, double‐blind, placebo‐controlled study. Vaccine 2005;23(43):5120‐6. - PubMed
McKenzie 2007 {published data only}
-
- McKenzie R, Bourgeois AL, Frech SA, Flyer DC, Bloom A, Kazempour K, et al. Transcutaneous immunization with the heat‐labile toxin (LT) of enterotoxigenic Escherichia coli (ETEC): protective efficacy in a double‐blind, placebo‐controlled challenge study. Vaccine 2007;25(18):3684‐91. - PubMed
McKenzie 2008 {published data only}
-
- McKenzie R, Darsley M, Thomas N, Randall R, Carpenter C, Forbes E, et al. A double‐blind, placebo‐controlled trial to evaluate the efficacy of PTL‐003, an attenuated enterotoxigenic E. coli (ETEC) vaccine strain, in protecting against challenge with virulent ETEC. Vaccine 2008;26(36):4731‐9. - PubMed
Peltola 1991 {published data only}
-
- Peltola H, Siitonen A, Kyrönseppä H, Simula I, Mattila L, Oksanen P, et al. Prevention of travellers' diarrhoea by oral B‐subunit/whole‐cell cholera vaccine. Lancet 1991;338(8778):1285‐9. - PubMed
Qadri 2003 {published data only}
-
- Qadri F, Ahmed T, Ahmed F, Bradley Sack R, Sack DA, Svennerholm AM. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi children 18‐36 months of age. Vaccine 2003;21(19‐20):2394‐403. - PubMed
Qadri 2006a {published data only}
-
- Qadri F, Ahmed T, Ahmed F, Begum Y A, Sack DA, Svennerholm AM, et al. Reduced doses of oral killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine is safe and immunogenic in Bangladeshi infants 6‐17 months of age: dosing studies in different age groups. Vaccine 2006;24(10):1726‐33. - PubMed
Sack 2007 {published data only}
-
- Sack DA, Shimko J, Torres O, Bourgeois AL, Francia DS, Gustafsson B, et al. Randomised, double‐blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhoea of travellers to Guatemala and Mexico. Vaccine 2007;25(22):4392‐400. - PubMed
Savarino 1998 {published data only}
-
- Savarino SJ, Brown FM, Hall E, Bassily S, Youssef F, Wierzba T, et al. Safety and immunogenicity of an oral, killed enterotoxigenic Escherichia coli‐cholera toxin B subunit vaccine in Egyptian adults. Journal of Infectious Diseases 1998;177(3):796‐9. - PubMed
Savarino 1999 (Study 1) {published data only}
-
- Savarino SJ, Hall ER, Bassily S, Brown FM, Youssef F, Wierzba TF, et al. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. PRIDE Study Group. Journal of Infectious Diseases 1999;179(1):107‐14. - PubMed
Savarino 1999 (Study 2) {published data only}
-
- Savarino SJ, Hall ER, Bassily S, Brown FM, Youssef F, Wierzba TF, et al. Oral, inactivated, whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine: results of the initial evaluation in children. PRIDE Study Group. Journal of Infectious Diseases 1999;179(1):107‐14. - PubMed
Savarino 2002 {published data only}
-
- Savarino SJ, Hall ER, Bassily S, Wierzba TF, Youssef FG, Peruski LF, et al. Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. Pediatric Infectious Disease Journal 2002;21(4):322‐30. - PubMed
Scerpella 1995 {published data only}
-
- Scerpella EG, Sanchez JL, Mathewson III JJ, Torres‐Cordero JV, Sadoff JC, Svennerholm AM, et al. Safety, immunogenicity, and protective efficacy of the whole‐cell/recombinant B subunit (WC/rBS) oral cholera vaccine against travelers' diarrhea. Journal of Travel Medicine 1995;2(1):22‐7. - PubMed
Tacket 1999 {published data only}
-
- Tacket CO, Losonsky G, Livio S, Edelman R, Crabb J, Freedman D. Lack of prophylactic efficacy of an enteric‐coated bovine hyperimmune milk product against enterotoxigenic Escherichia coli challenge administered during a standard meal. Journal of Infectious Diseases 1999;180(6):2056‐9. - PubMed
Wiedermann 2000 {published data only}
-
- Wiedermann G, Kollaritsch H, Kundi M, Svennerholm AM, Bjare U. Double‐blind, randomized, placebo controlled pilot study evaluating efficacy and reactogenicity of an oral ETEC B‐subunit‐inactivated whole cell vaccine against travelers' diarrhea (preliminary report). Journal of Travel Medicine 2000;7(1):27‐9. - PubMed
References to studies excluded from this review
Ahmed 2006 {published data only}
-
- Ahmed A, Li J, Shiloach Y, Bobbins JB, Szu SC. Safety and immunogenicity of Escherichia coli O157 O‐specific polysaccharide conjugate vaccine in 2‐5‐year‐old children. Journal of Infectious Diseases 2006;193(4):515‐21. - PubMed
Ahren 1998 {published data only}
Carpenter 2006 {published data only}
-
- Carpenter CM, Hall ER, Randall R, McKenzie R, Cassels F, Diaz N, et al. Comparison of the antibody in lymphocyte supernatant (ALS) and ELISPOT assays for detection of mucosal immune responses to antigens of enterotoxigenic Escherichia coli in challenged and vaccinated volunteers. Vaccine 2006;24(18):3709‐18. - PubMed
Clemens 2004 {published data only}
-
- Clemens J, Savarino S, Abu‐Elyazeed R, Safwat M, Rao M, Wierzba T, et al. Development of pathogenicity‐driven definitions of outcomes for a field trial of a killed oral vaccine against enterotoxigenic Escherichia coli in Egypt: application of an evidence‐based method . Journal of Infectious Disease 2004;189(12):2299‐307. - PubMed
Coster 2007 {published data only}
Daley 2007 {published data only}
Evans 1984 {published data only}
-
- Evans DG, Graham DY, Evans DJ Jr. Administration of purified colonization factor antigens (CFA/I, CFA/II) of enterotoxigenic Escherichia coli to volunteers. Response to challenge with virulent enterotoxigenic Escherichia coli. Gastroenterology 1984;87(4):934‐40. - PubMed
Evans 1988a {published data only}
-
- Evans DG, Evans DJ Jr, Opekun AR, Graham DY. Non‐replicating oral whole cell vaccine protective against enterotoxigenic Escherichia coli (ETEC) diarrhea: stimulation of anti‐CFA (CFA/I) and anti‐enterotoxin (anti‐LT) intestinal IgA and protection against challenge with ETEC belonging to heterologous serotypes. FEMS Microbiology Immunology 1988;1(3):117‐26. - PubMed
Evans 1988b {published data only}
-
- Evans DJ, Evans DG, Opekun AR, Graham DY. Immunoprotective oral whole cell vaccine for enterotoxigenic Escherichia coli diarrhea prepared by in situ destruction of chromosomal and plasmid DNA with colicin E2. FEMS Microbiology Immunology 1988;1(1):9‐18. - PubMed
Glenn 2007 {published data only}
Guereña‐Burgueño 2002 {published data only}
Hallander 2002 {published data only}
-
- Hallander HO, Paniagua M, Espinoza F, Askelöf P, Corrales E, Ringman M, Storsaeter J. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine 2002;22(1‐2):138‐45. - PubMed
Holmgren 1992 {published data only}
-
- Holmgren J, Svennerholm AM, Jertborn M, Clemens J, Sack DA, Salenstedt R, et al. An oral B subunit: whole cell vaccine against cholera. Vaccine 1992;10(13):911‐4. - PubMed
Katz 2003 {published data only}
-
- Katz DE, DeLorimier AJ, Wolf MK, Hall ER, Cassels FJ, Hamont JE, et al. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine 2003;21(5‐6):341‐6. - PubMed
Khan 2007 {published data only}
Klipstein 1986 {published data only}
-
- Klipstein FA, Engert RF, Houghten RA. Immunisation of volunteers with a synthetic peptide vaccine for enterotoxigenic Escherichia coli. Lancet 1986;1(8479):471‐2. - PubMed
Lapa 2008 {published data only}
-
- Lapa JA, Sincock SA, Ananthakrishnan M, Porter CK, Cassels FJ, Brinkley C, et al. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat‐labile enterotoxin with mutation R192G. Clinical and Vaccine Immunology 2008;15(8):1222‐8. - PMC - PubMed
Levine 1982 {published data only}
-
- Levine MM, Black RE, Brinton CC Jr, Clements ML, Fusco P, Hughes TP, et al. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scandinavian Journal of Infectious Diseases 1982;33:83‐95. - PubMed
McKenzie 2006 {published data only}
Qadri 2006b {published data only}
Sougioultzis 2002 {published data only}
-
- Sougioultzis S, Lee CK, Alsahli M, Banerjee S, Cadoz M, Schrader R, et al. Safety and efficacy of E coli enterotoxin adjuvant for urease‐based rectal immunization against Helicobacter pylori. Vaccine 2002;21(3‐4):194‐201. - PubMed
Tacket 1994 {published data only}
-
- Tacket CO, Reid RH, Boedeker EC, Losonsky G, Nataro JP, Bhagat H, et al. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine 1994;12(14):1270‐4. - PubMed
Turner 2006 {published data only}
-
- Turner AK, Beavis JC, Stephens JC, Greenwood J, Gewert C, Thomas N, et al. Construction and phase I clinical evaluation of the safety and immunogenicity of a candidate enterotoxigenic Escherichia coli vaccine strain expressing colonization factor antigen CFA/I. Infection and immunity 2006;74(2):1062‐71. - PMC - PubMed
Wasserman 1993 {published data only}
-
- Wasserman SS, Kotloff KL, Losonsky GA, Levine MM. Immunologic response to oral cholera vaccination in a crossover study: a novel placebo effect. American Journal of Epidemiology 1993;138(11):988‐93. - PubMed
Additional references
Black 1984
-
- Black RE, Merson MH, Eusof A, Huq I, Pollard R. Nutritional status, body size and severity of diarrhoea associated with rotavirus or enterotoxigenic Escherichia coli. Journal of Tropical Medicine & Hygiene 1984;87(2):8309. - PubMed
Darsley 2012
-
- Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, et al. The oral, live attenuated enterotoxigenic Eschericia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clinical and Vaccine Immunology 2012;19(12):1921‐31. - PMC - PubMed
DuPont 2008
-
- DuPont HL. Systematic review: prevention of travellers' diarrhoea. Alimentary Pharmacology & Therapeutics 2008;27(9):741‐51. - PubMed
Gill 1980
-
- Gill DM, Richardson SH. Adenosine diphosphate‐ribosylation of adenylate cyclase catalyzed by heat‐labile enterotoxin of Escherichia coli: comparison with cholera toxin. Journal of Infectious Diseases 1980;141(1):64‐70. - PubMed
Guyatt 2008
Harris 2008
-
- Harris AM, Chowdhury F, Begum YA, Khan AI, Faruque AS, Svennerholm AM, et al. Shifting prevalence of major diarrheal pathogens in patients seeking hospital care during floods in 1998, 2004, and 2007 in Dhaka, Bangladesh. American Journal of Tropical Medicine and Hygiene 2008;79(5):708‐14. - PMC - PubMed
Harro 2011
-
- Harro C, Sack D, Bourgeois AL, Walker R, DeNearing B, Feller A, et al. A combination vaccine consisting of three live attenuated enterotoxigenic Escherichia coli strains expressing a range of colonization factors and heat‐labile toxin subunit B is well tolerated and immunogenic in a placebo‐controlled double‐blind phase I trial in healthy adults. Clinical and Vaccine Immunology 2011;18(12):2118‐27. - PMC - PubMed
Higgins 2008
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008.
Holmgren 2005
-
- Holmgren J, Adamsson J, Anjuere F, Clemens J, Czerkinsky C, Eriksson K, et al. Mucosal adjuvants and anti‐infection and anti‐immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunology Letters 2005;97(2):181‐8. - PubMed
Holmgren 2012
-
- Holmgren J, Svennerholm A. Vaccines against mucosal infections. Current Opinion in Immunology 2012;24(3):343‐53. - PubMed
Jelinek 2008
-
- Jelinek T, Kollaritsch H. Vaccination with Dukoral against travelers' diarrhea (ETEC) and cholera. Expert Review of Vaccines 2008;7(5):561‐7. - PubMed
Jertborn 1992
-
- Jertborn M, Svennerholm A‐M, Holmgren J. Safety and immunogenicity of an oral recombinant cholera b subunit‐whole cell vaccine in Swedish volunteers. Vaccine 1992;10(2):130‐2. - PubMed
Qadri 2005
Qadri 2007
Rao 2005
-
- Rao MR, Wierzba TF, Savarino SJ, Abu‐Elyazeed R, El‐Ghoreb N, Hall ER, et al. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. Journal of Infectious Diseases 2005;191(4):562‐70. - PubMed
Review Manager (RevMan) [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schwartz 2006
Steffen 2005
-
- Steffen R, Castelli F, Dieter Nothdurft H, Rombo L, Jane Zuckerman N. Vaccination against enterotoxigenic Escherichia coli, a cause of travelers' diarrhea. Journal of Travel Medicine 2005;12(2):102‐7. - PubMed
Svennerholm 1984
-
- Svennerholm AM, Jertborn M, Gothefors L, Karim AM, Sack DA, Holmgren J. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit‐whole cell vaccine. Journal of Infectious Diseases 1984;149(6):884‐93. - PubMed
Svennerholm 2008
-
- Svennerholm AM, Tobias J. Vaccines against enterotoxigenic Escherichia coli. Expert Review of Vaccines 2008;7(6):795‐804. - PubMed
Tobias 2011
-
- Tobias J, Svennerholm AM, Carlin NI, Lebens M, Holmgren J. Construction of a non‐toxigenic Escherichia coli oral vaccine strain expressing large amounts of CS6 and inducing strong intestinal and serum anti‐CS6 antibody responses in mice. Vaccine 2011;29(48):8863‐9. - PubMed
Tobias 2012
-
- Tobias J, Svennerholm AM. Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole‐cell inactivated ETEC vaccine candidates. Applied Microbiology and Biotechnology 2012;93(6):2291‐300. - PubMed
Walker 2007
-
- Walker RI, Steele D, Aguado T, Ad Hoc ETEC Technical Expert Committee. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 2007;25(14):2545‐66. - PubMed
Wennerås 2004
-
- Wennerås C, Erling V. Prevalence of enterotoxigenic Escherichia coli‐associated diarrhoea and carrier state in the developing world. Journal of Health, Population and Nutrition 2004;22(4):370‐82. - PubMed
WHO 2009
-
- WHO. Diarrhoeal diseases (updated February 2009). http://www.who.int/vaccine_research/diseases/diarrhoeal/en/index4.html 2009.
Widermann 2009
-
- Wagner A, Wiedermann U. Travellers’ diarrhoea – pros and cons of different prophylactic measures. Wiener Klinische Wochenschrift 2009;121(Suppl 3):13‐8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

