Phosphoproteomic analysis reveals the effects of PilF phosphorylation on type IV pilus and biofilm formation in Thermus thermophilus HB27
- PMID: 23828892
- PMCID: PMC3790284
- DOI: 10.1074/mcp.M113.029330
Phosphoproteomic analysis reveals the effects of PilF phosphorylation on type IV pilus and biofilm formation in Thermus thermophilus HB27
Abstract
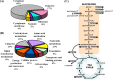
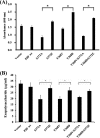
Thermus thermophilus HB27 is an extremely thermophilic eubacteria with a high frequency of natural competence. This organism is therefore often used as a thermophilic model to investigate the molecular basis of type IV pili-mediated functions, such as the uptake of free DNA, adhesion, twitching motility, and biofilm formation, in hot environments. In this study, the phosphoproteome of T. thermophilus HB27 was analyzed via a shotgun approach and high-accuracy mass spectrometry. Ninety-three unique phosphopeptides, including 67 in vivo phosphorylated sites on 53 phosphoproteins, were identified. The distribution of Ser/Thr/Tyr phosphorylation sites was 57%/36%/7%. The phosphoproteins were mostly involved in central metabolic pathways and protein/cell envelope biosynthesis. According to this analysis, the ATPase motor PilF, a type IV pili-related component, was first found to be phosphorylated on Thr-368 and Ser-372. Through the point mutation of PilF, mimic phosphorylated mutants T368D and S372E resulted in nonpiliated and nontwitching phenotypes, whereas nonphosphorylated mutants T368V and S372A displayed piliation and twitching motility. In addition, mimic phosphorylated mutants showed elevated biofilm-forming abilities with a higher initial attachment rate, caused by increasing exopolysaccharide production. In summary, the phosphorylation of PilF might regulate the pili and biofilm formation associated with exopolysaccharide production.
Figures


Similar articles
-
Type IV pilus biogenesis, twitching motility, and DNA uptake in Thermus thermophilus: discrete roles of antagonistic ATPases PilF, PilT1, and PilT2.Appl Environ Microbiol. 2014 Jan;80(2):644-52. doi: 10.1128/AEM.03218-13. Epub 2013 Nov 8. Appl Environ Microbiol. 2014. PMID: 24212586 Free PMC article.
-
Molecular analyses of the natural transformation machinery and identification of pilus structures in the extremely thermophilic bacterium Thermus thermophilus strain HB27.Appl Environ Microbiol. 2002 Feb;68(2):745-55. doi: 10.1128/AEM.68.2.745-755.2002. Appl Environ Microbiol. 2002. PMID: 11823215 Free PMC article.
-
Zinc and ATP binding of the hexameric AAA-ATPase PilF from Thermus thermophilus: role in complex stability, piliation, adhesion, twitching motility, and natural transformation.J Biol Chem. 2014 Oct 31;289(44):30343-30354. doi: 10.1074/jbc.M114.598656. Epub 2014 Sep 8. J Biol Chem. 2014. PMID: 25202014 Free PMC article.
-
Type IV Pili in Thermophilic Bacteria: Mechanisms and Ecological Implications.Biomolecules. 2025 Mar 21;15(4):459. doi: 10.3390/biom15040459. Biomolecules. 2025. PMID: 40305182 Free PMC article. Review.
-
Shuffling genes around in hot environments: the unique DNA transporter of Thermus thermophilus.FEMS Microbiol Rev. 2009 May;33(3):611-26. doi: 10.1111/j.1574-6976.2008.00160.x. Epub 2009 Jan 16. FEMS Microbiol Rev. 2009. PMID: 19207744 Review.
Cited by
-
High-efficiency decomposition of eggshell membrane by a keratinase from Meiothermus taiwanensis.Sci Rep. 2022 Aug 29;12(1):14684. doi: 10.1038/s41598-022-18474-4. Sci Rep. 2022. PMID: 36038640 Free PMC article.
-
Design, Co-Expression, and Evaluation for Assembly of the Structural Proteins from Thermophilic Bacteriophage ΦIN93.Int J Mol Sci. 2025 May 28;26(11):5201. doi: 10.3390/ijms26115201. Int J Mol Sci. 2025. PMID: 40508011 Free PMC article.
-
Cyclic-di-GMP and ADP bind to separate domains of PilB as mutual allosteric effectors.Biochem J. 2020 Jan 17;477(1):213-226. doi: 10.1042/BCJ20190809. Biochem J. 2020. PMID: 31868878 Free PMC article.
-
Lysine propionylation is a prevalent post-translational modification in Thermus thermophilus.Mol Cell Proteomics. 2014 Sep;13(9):2382-98. doi: 10.1074/mcp.M113.035659. Epub 2014 Jun 17. Mol Cell Proteomics. 2014. PMID: 24938286 Free PMC article.
-
Comparative Phosphoproteomics Reveals the Role of AmpC β-lactamase Phosphorylation in the Clinical Imipenem-resistant Strain Acinetobacter baumannii SK17.Mol Cell Proteomics. 2016 Jan;15(1):12-25. doi: 10.1074/mcp.M115.051052. Epub 2015 Oct 23. Mol Cell Proteomics. 2016. PMID: 26499836 Free PMC article.
References
-
- Oshima T. (1974) Comparative studies on biochemical properties of an extreme thermophile, Thermus thermophilus HB 8. Seikagaku 46, 887–907 - PubMed
-
- Pantazaki A. A., Pritsa A. A., Kyriakidis D. A. (2002) Biotechnologically relevant enzymes from Thermus thermophilus. Appl. Microbiol. Biotechnol. 58, 1–12 - PubMed
-
- Cava F., Hidalgo A., Berenguer J. (2009) Thermus thermophilus as biological model. Extremophiles 13, 213–231 - PubMed
-
- Degryse E., Glansdorff N., Pierard A. (1978) Comparative analysis of extreme thermophilic bacteria belonging to genus Thermus. Archiv. Microbiol. 117, 189–196 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

