Latent myostatin has significant activity and this activity is controlled more efficiently by WFIKKN1 than by WFIKKN2
- PMID: 23829672
- PMCID: PMC3906830
- DOI: 10.1111/febs.12377
Latent myostatin has significant activity and this activity is controlled more efficiently by WFIKKN1 than by WFIKKN2
Abstract
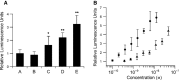
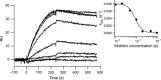
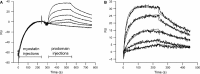
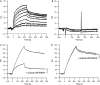
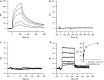
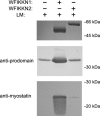
Myostatin, a negative regulator of skeletal muscle growth, is produced from myostatin precursor by multiple steps of proteolytic processing. After cleavage by a furin-type protease, the propeptide and growth factor domains remain associated, forming a noncovalent complex, the latent myostatin complex. Mature myostatin is liberated from latent myostatin by bone morphogenetic protein 1/tolloid proteases. Here, we show that, in reporter assays, latent myostatin preparations have significant myostatin activity, as the noncovalent complex dissociates at an appreciable rate, and both mature and semilatent myostatin (a complex in which the dimeric growth factor domain interacts with only one molecule of myostatin propeptide) bind to myostatin receptor. The interaction of myostatin receptor with semilatent myostatin is efficiently blocked by WAP, Kazal, immunoglobulin, Kunitz and NTR domain-containing protein 1 or growth and differentiation factor-associated serum protein 2 (WFIKKN1), a large extracellular multidomain protein that binds both mature myostatin and myostatin propeptide [Kondás et al. (2008) J Biol Chem 283, 23677-23684]. Interestingly, the paralogous protein WAP, Kazal, immunoglobulin, Kunitz and NTR domain-containing protein 2 or growth and differentiation factor-associated serum protein 1 (WFIKKN2) was less efficient than WFIKKN1 as an antagonist of the interactions of myostatin receptor with semilatent myostatin. Our studies have shown that this difference is attributable to the fact that only WFIKKN1 has affinity for the propeptide domain, and this interaction increases its potency in suppressing the receptor-binding activity of semilatent myostatin. As the interaction of WFIKKN1 with various forms of myostatin permits tighter control of myostatin activity until myostatin is liberated from latent myostatin by bone morphogenetic protein 1/tolloid proteases, WFIKKN1 may have greater potential as an antimyostatic agent than WFIKKN2.
Keywords: WFIKKN1; WFIKKN2; latent myostatin; myostatin; promyostatin.
© 2013 The Authors. FEBS Journal published by John Wiley & Sons Ltd on behalf of FEBS.
Figures




Similar articles
-
Both WFIKKN1 and WFIKKN2 have high affinity for growth and differentiation factors 8 and 11.J Biol Chem. 2008 Aug 29;283(35):23677-84. doi: 10.1074/jbc.M803025200. Epub 2008 Jul 1. J Biol Chem. 2008. PMID: 18596030 Free PMC article.
-
Influence of WFIKKN1 on BMP1-mediated activation of latent myostatin.FEBS J. 2016 Dec;283(24):4515-4527. doi: 10.1111/febs.13938. Epub 2016 Nov 16. FEBS J. 2016. PMID: 27782377
-
Biological functions of the WAP domain-containing multidomain proteins WFIKKN1 and WFIKKN2.Biochem Soc Trans. 2011 Oct;39(5):1416-20. doi: 10.1042/BST0391416. Biochem Soc Trans. 2011. PMID: 21936825 Review.
-
Crystal structure of the WFIKKN2 follistatin domain reveals insight into how it inhibits growth differentiation factor 8 (GDF8) and GDF11.J Biol Chem. 2019 Apr 19;294(16):6333-6343. doi: 10.1074/jbc.RA118.005831. Epub 2019 Feb 27. J Biol Chem. 2019. PMID: 30814254 Free PMC article.
-
WFIKKN1 and WFIKKN2: "Companion" proteins regulating TGFB activity.Cytokine Growth Factor Rev. 2016 Dec;32:75-84. doi: 10.1016/j.cytogfr.2016.06.003. Epub 2016 Jun 11. Cytokine Growth Factor Rev. 2016. PMID: 27325460 Review.
Cited by
-
Alternative binding modes identified for growth and differentiation factor-associated serum protein (GASP) family antagonism of myostatin.J Biol Chem. 2015 Mar 20;290(12):7506-16. doi: 10.1074/jbc.M114.624130. Epub 2015 Feb 5. J Biol Chem. 2015. PMID: 25657005 Free PMC article.
-
Analysis of PI3K pathway components in human cancers.Oncol Lett. 2016 Apr;11(4):2913-2918. doi: 10.3892/ol.2016.4309. Epub 2016 Mar 8. Oncol Lett. 2016. PMID: 27073576 Free PMC article.
-
A targeted proteomic assay for the measurement of plasma proteoforms related to human aging phenotypes.Proteomics. 2017 Aug;17(15-16):10.1002/pmic.201600232. doi: 10.1002/pmic.201600232. Proteomics. 2017. PMID: 28508553 Free PMC article.
-
Agonists and Antagonists of TGF-β Family Ligands.Cold Spring Harb Perspect Biol. 2016 Aug 1;8(8):a021923. doi: 10.1101/cshperspect.a021923. Cold Spring Harb Perspect Biol. 2016. PMID: 27413100 Free PMC article. Review.
-
Dynamic allostery: a novel mechanism regulating autocrine and paracrine TGF-β signalling.Signal Transduct Target Ther. 2025 Jan 3;10(1):3. doi: 10.1038/s41392-024-02099-2. Signal Transduct Target Ther. 2025. PMID: 39746900 Free PMC article. No abstract available.
References
-
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. - PubMed
-
- Szabó G, Dallmann G, Müller G, Patthy L, Soller M, Varga L. A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome. 1998;9:671–672. - PubMed
-
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Ménissier F, Massabanda J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet. 1997;17:71–74. - PubMed
-
- Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

