CD8(+) T cells in multiple sclerosis
- PMID: 23829711
- PMCID: PMC3928018
- DOI: 10.1517/14728222.2013.815726
CD8(+) T cells in multiple sclerosis
Abstract
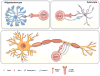
Introduction: CD8(+) T cells were originally considered to exert a suppressive role in demyelinating disease because of bias toward the CD4(+) T cell-mediated experimental autoimmune encephalomyelitis, the most common multiple sclerosis (MS) model. However, recent studies of human MS lesion samples and cerebrospinal fluid (CSF) provided compelling evidence about the pathogenic role of CD8(+) T cells. In this article, we discuss the theoretical roles of different CD8(+) T-cell subsets in MS.
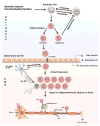
Areas covered: A revised focus from CD4(+) to CD8(+) T cell-mediated demyelinating disease is summarized. Clonal expansion of CD8(+) T cells in MS lesions and in vitro evidence that CD8(+) T cells injure every central nervous system (CNS) cell type and transect axons are discussed. The role of CD8(+) T cells in two animal models of MS and of regulatory, interleukin (IL)-17-secreting CD8(+) T cells is reviewed. Lastly, an overview about the pathogenic and/or beneficial role of various CD8(+) T-cell subsets is offered.
Expert opinion: Growing evidence supports the pathogenic role of CD8(+) T cells. Clonally expanded CD8(+) T cells within MS lesions may damage the nervous system. Revealing the specific antigen is critical to design novel efficient treatments with minimal adverse effects. Increasing evidence exists for the role of regulatory, IL-17-secreting CD8(+) T cells in MS.
Figures


References
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, et al. Multiple sclerosis. N Engl J Med. 2000;343:938–52. - PubMed
-
- Kappos L, Comi G, Panitch H, et al. Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med. 2000;6:1176–82. - PubMed
-
- Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med. 2000;6:1167–75. - PubMed
-
- Rivera-Quinones C, McGavern D, Schmelzer JD, et al. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat Med. 1998;4:187–93. Important study that clearly implicates CD8+ T cells as pathogenic, leading to axonal loss and clinical deficits in a murine model of demyelination. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
