Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function
- PMID: 23830147
- PMCID: PMC3785237
- DOI: 10.1016/j.jaci.2013.05.029
Signal transducer and activator of transcription 3 (STAT3) mutations underlying autosomal dominant hyper-IgE syndrome impair human CD8(+) T-cell memory formation and function
Abstract
Background: The capacity of CD8(+) T cells to control infections and mediate antitumor immunity requires the development and survival of effector and memory cells. IL-21 has emerged as a potent inducer of CD8(+) T-cell effector function and memory development in mouse models of infectious disease. However, the role of IL-21 and associated signaling pathways in protective CD8(+) T-cell immunity in human subjects is unknown.
Objective: We sought to determine which signaling pathways mediate the effects of IL-21 on human CD8(+) T cells and whether defects in these pathways contribute to disease pathogenesis in patients with primary immunodeficiencies caused by mutations in components of the IL-21 signaling cascade.
Methods: Human primary immunodeficiencies resulting from monogenic mutations provide a unique opportunity to assess the requirement for particular molecules in regulating human lymphocyte function. Lymphocytes from patients with loss-of-function mutations in signal transducer and activator of transcription 1 (STAT1), STAT3, or IL-21 receptor (IL21R) were used to assess the respective roles of these genes in human CD8(+) T-cell differentiation in vivo and in vitro.
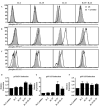
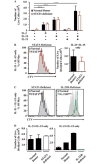
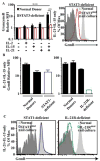
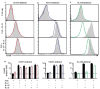
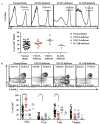
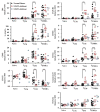
Results: Mutations in STAT3 and IL21R, but not STAT1, led to a decrease in multiple memory CD8(+) T-cell subsets in vivo, indicating that STAT3 signaling, possibly downstream of IL-21R, regulates the memory cell pool. Furthermore, STAT3 was important for inducing the lytic machinery in IL-21-stimulated naive CD8(+) T cells. However, this defect was overcome by T-cell receptor engagement.
Conclusion: The IL-21R/STAT3 pathway is required for many aspects of human CD8(+) T-cell behavior but in some cases can be compensated by other signals. This helps explain the relatively mild susceptibility to viral disease observed in STAT3- and IL-21R-deficient subjects.
Keywords: AD-HIES; Autosomal dominant hyper-IgE syndrome; B-cell lymphoma; BCL; CTV; CellTrace Violet; Central memory T; EOMES; Effector memory T; Effector memory T cells expressing CD45RA; Eomesodermin; IL-21; IL-21 receptor; IL-21R; LCMV; Lymphocytic choriomeningitis virus; PB; PID; Peripheral blood; Primary immunodeficiency; STAT; STAT1; STAT3; Signal transducer and activator of transcription; T(CM); T(EM); T(EMRA); T-cell receptor; TCR; differentiation; human CD8(+) T cells; memory.
Published by Mosby, Inc.
Conflict of interest statement
The authors declare no conflicts of interest
Figures

Comment in
-
Patients informing immunobiology: How disorders of IL-21 receptor signaling unravel pathways of CD8 T-cell function.J Allergy Clin Immunol. 2013 Aug;132(2):412-3. doi: 10.1016/j.jaci.2013.06.024. J Allergy Clin Immunol. 2013. PMID: 23905919 Free PMC article. No abstract available.
References
-
- Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev. 2002;13:429–39. - PubMed
-
- Surh CD, Sprent J. Homeostasis of Naive and Memory T Cells. Immunity. 2008;29:848–62. - PubMed
-
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged Interleukin-2Rα Expression on Virus-Specific CD8+ T Cells Favors Terminal-Effector Differentiation In Vivo. Immunity. 2010;32:91–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

