MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling
- PMID: 23830207
- PMCID: PMC3767157
- DOI: 10.1016/j.cell.2013.06.026
MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling
Abstract
Tumor cells metastasize to distant organs through genetic and epigenetic alterations, including changes in microRNA (miR) expression. Here we find miR-22 triggers epithelial-mesenchymal transition (EMT), enhances invasiveness and promotes metastasis in mouse xenografts. In a conditional mammary gland-specific transgenic (TG) mouse model, we show that miR-22 enhances mammary gland side-branching, expands the stem cell compartment, and promotes tumor development. Critically, miR-22 promotes aggressive metastatic disease in MMTV-miR-22 TG mice, as well as compound MMTV-neu or -PyVT-miR-22 TG mice. We demonstrate that miR-22 exerts its metastatic potential by silencing antimetastatic miR-200 through direct targeting of the TET (Ten eleven translocation) family of methylcytosine dioxygenases, thereby inhibiting demethylation of the mir-200 promoter. Finally, we show that miR-22 overexpression correlates with poor clinical outcomes and silencing of the TET-miR-200 axis in patients. Taken together, our findings implicate miR-22 as a crucial epigenetic modifier and promoter of EMT and breast cancer stemness toward metastasis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
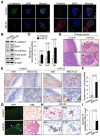
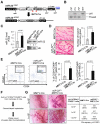


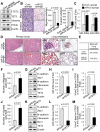
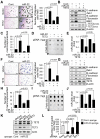

Comment in
-
Oncogenic miRNAs and the perils of losing control of a stem cell's epigenetic identity.Cell Stem Cell. 2013 Jul 3;13(1):5-6. doi: 10.1016/j.stem.2013.06.012. Cell Stem Cell. 2013. PMID: 23827702
-
miR-22 in tumorigenesis.Cell Cycle. 2014;13(1):11-2. doi: 10.4161/cc.27027. Epub 2013 Nov 14. Cell Cycle. 2014. PMID: 24231768 Free PMC article. No abstract available.
References
-
- Adam L, Zhong M, Choi W, Qi W, Nicoloso M, Arora A, Calin G, Wang H, Siefker-Radtke A, McConkey D, et al. miR-200 expression regulates epithelial-to-mesenchymal transition in bladder cancer cells and reverses resistance to epidermal growth factor receptor therapy. Clin. Cancer Res. 2009;15:5060–5072. - PMC - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
-
- Bendoraite A, Knouf EC, Garg KS, Parkin RK, Kroh EM, O'Briant KC, Ventura AP, Godwin AK, Karlan BY, Drescher CW, et al. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: evidence supporting a mesothelial-to-epithelial transition. Gynecol. Oncol. 2010;116:117–125. - PMC - PubMed
-
- Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–153. microRNA.org - PMC - PubMed
-
- Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer. 2006;6:506–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

