Intersecting circuits generate precisely patterned retinal waves
- PMID: 23830830
- PMCID: PMC3737599
- DOI: 10.1016/j.neuron.2013.05.012
Intersecting circuits generate precisely patterned retinal waves
Abstract
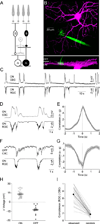
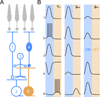
The developing retina generates spontaneous glutamatergic (stage III) waves of activity that sequentially recruit neighboring ganglion cells with opposite light responses (ON and OFF RGCs). This activity pattern is thought to help establish parallel ON and OFF pathways in downstream visual areas. The circuits that produce stage III waves and desynchronize ON and OFF RGC firing remain obscure. Using dual patch-clamp recordings, we find that ON and OFF RGCs receive sequential excitatory input from ON and OFF cone bipolar cells (CBCs), respectively. This input sequence is generated by crossover circuits, in which ON CBCs control glutamate release from OFF CBCs via diffusely stratified inhibitory amacrine cells. In addition, neighboring ON CBCs communicate directly and indirectly through lateral glutamatergic transmission and gap junctions, both of which are required for wave initiation and propagation. Thus, intersecting lateral excitatory and vertical inhibitory circuits give rise to precisely patterned stage III retinal waves.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

