Long-term effects of a lumbosacral ventral root avulsion injury on axotomized motor neurons and avulsed ventral roots in a non-human primate model of cauda equina injury
- PMID: 23830908
- PMCID: PMC3888796
- DOI: 10.1016/j.neuroscience.2013.06.054
Long-term effects of a lumbosacral ventral root avulsion injury on axotomized motor neurons and avulsed ventral roots in a non-human primate model of cauda equina injury
Abstract
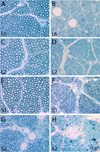
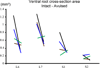
Here, we have translated from the rat to the non-human primate a unilateral lumbosacral injury as a model for cauda equina injury. In this morphological study, we have investigated retrograde effects of a unilateral L6-S2 ventral root avulsion (VRA) injury as well as the long-term effects of Wallerian degeneration on avulsed ventral roots at 6-10 months post-operatively in four adult male rhesus monkeys. Immunohistochemistry for choline acetyl transferase and glial fibrillary acidic protein demonstrated a significant loss of the majority of the axotomized motoneurons in the affected L6-S2 segments and signs of an associated astrocytic glial response within the ventral horn of the L6 and S1 spinal cord segments. Quantitative analysis of the avulsed ventral roots showed that they exhibited normal size and were populated by a normal number of myelinated axons. However, the myelinated axons in the avulsed ventral roots were markedly smaller in caliber compared to the fibers of the intact contralateral ventral roots, which served as controls. Ultrastructural studies confirmed the presence of small myelinated axons and a population of unmyelinated axons within the avulsed roots. In addition, collagen fibers were readily identified within the endoneurium of the avulsed roots. In summary, a lumbosacral VRA injury resulted in retrograde motoneuron loss and astrocytic glial activation in the ventral horn. Surprisingly, the Wallerian degeneration of motor axons in the avulsed ventral roots was followed by a repopulation of the avulsed roots by small myelinated and unmyelinated fibers. We speculate that the small axons may represent sprouting or axonal regeneration by primary afferents or autonomic fibers.
Keywords: ChAT; GFAP; PBS; VRA; Wallerian degeneration; choline acetyltransferase; electron microscopy; glial fibrillary acidic protein; phosphate-buffered saline; rhesus macaque; spinal cord; ventral root avulsion.
Copyright © 2013 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures








Similar articles
-
Glial reactions in a rodent cauda equina injury and repair model.Exp Brain Res. 2006 Mar;170(1):52-60. doi: 10.1007/s00221-005-0188-6. Epub 2005 Nov 17. Exp Brain Res. 2006. PMID: 16328291
-
Acute implantation of an avulsed lumbosacral ventral root into the rat conus medullaris promotes neuroprotection and graft reinnervation by autonomic and motor neurons.Neuroscience. 2006;138(4):1149-60. doi: 10.1016/j.neuroscience.2005.11.066. Epub 2006 Jan 30. Neuroscience. 2006. PMID: 16446042
-
Reimplantation of avulsed lumbosacral ventral roots in the rat ameliorates injury-induced degeneration of primary afferent axon collaterals in the spinal dorsal columns.Neuroscience. 2008 Mar 18;152(2):338-45. doi: 10.1016/j.neuroscience.2007.11.043. Epub 2007 Dec 8. Neuroscience. 2008. PMID: 18291596 Free PMC article.
-
Functional recovery after ventral root avulsion and implantation in the spinal cord.Clin Neurol Neurosurg. 1993;95 Suppl:S109-11. doi: 10.1016/0303-8467(93)90046-j. Clin Neurol Neurosurg. 1993. PMID: 8467587 Review.
-
Experimental studies on surgical treatment of avulsed spinal nerve roots in brachial plexus injury.J Hand Surg Br. 1991 Dec;16(5):477-82. doi: 10.1016/0266-7681(91)90098-9. J Hand Surg Br. 1991. PMID: 1791354 Review.
Cited by
-
ISP and PAP4 peptides promote motor functional recovery after peripheral nerve injury.Neural Regen Res. 2021 Aug;16(8):1598-1605. doi: 10.4103/1673-5374.294565. Neural Regen Res. 2021. PMID: 33433490 Free PMC article.
-
Effects of local and systemic treatment with human natural killer-1 mimetic peptide (HNK-1) after ventral root avulsion and reimplantation in mice.J Venom Anim Toxins Incl Trop Dis. 2024 May 20;30:e20230065. doi: 10.1590/1678-9199-JVATITD-2023-0065. eCollection 2024. J Venom Anim Toxins Incl Trop Dis. 2024. PMID: 38770186 Free PMC article.
-
Up-regulation of heat shock protein 27 inhibits apoptosis in lumbosacral nerve root avulsion-induced neurons.Sci Rep. 2019 Aug 7;9(1):11468. doi: 10.1038/s41598-019-48003-9. Sci Rep. 2019. PMID: 31391542 Free PMC article.
-
GDNF Gene Therapy to Repair the Injured Peripheral Nerve.Front Bioeng Biotechnol. 2020 Oct 30;8:583184. doi: 10.3389/fbioe.2020.583184. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 33251197 Free PMC article. Review.
-
Selected gene profiles of stressed NSC-34 cells and rat spinal cord following peripheral nerve reconstruction and minocycline treatment.Exp Ther Med. 2016 May;11(5):1685-1699. doi: 10.3892/etm.2016.3130. Epub 2016 Mar 2. Exp Ther Med. 2016. PMID: 27168790 Free PMC article.
References
-
- Aldskogius H, Liu L, Svensson M. Glial responses to synaptic damage and plasticity. J. Neurosci. Res. 1999;58:33–41. - PubMed
-
- Armstrong DM, Brady R, Hersh LB, Hayes RC, Wiley RG. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. J. Comp Neurol. 304:596–607. - PubMed
-
- Bellander BM, Singhrao SK, Ohlsson M, Mattsson P, Svensson M. Complement activation in the human brain after traumatic head injury. J. Neurotrauma. 2001;18:1295–1311. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

