Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses
- PMID: 23831594
- PMCID: PMC3978791
- DOI: 10.1038/mt.2013.162
Coadministration of seasonal influenza vaccine and MVA-NP+M1 simultaneously achieves potent humoral and cell-mediated responses
Abstract
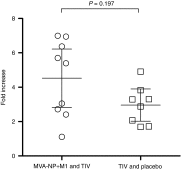
Current seasonal influenza vaccines have reduced immunogenicity and are of suboptimal efficacy in older adults. We have previously shown that the novel candidate vaccine MVA-NP+M1 is able to boost memory T cell responses in adults aged 50-85 years. Preclinical studies have demonstrated that viral vectored vaccines can act as adjuvants when coadministered with protein-based vaccines. We have conducted a phase I clinical trial to compare the coadministration of seasonal influenza vaccine and MVA-NP+M1 with seasonal influenza vaccine alone in adults aged 50 years and above. This combination of vaccines was safe and well tolerated. T cell responses to internal influenza proteins were boosted to significantly higher levels in the group receiving MVA-NP+M1 compared with the group receiving seasonal influenza vaccine alone. Rates of seroprotection and seroconversion against the three vaccine strains were similar in both groups; however, there was a significant increase in the geometric mean titer ratio for the H3N2 component of seasonal influenza vaccine in the coadministration group. While some vaccine combinations result in immune interference, the coadministration of MVA-NP+M1 alongside seasonal influenza vaccine is shown here to increase some influenza strain-specific antibody responses and boost memory T cells capable of recognizing a range of influenza A subtypes.
Figures


References
-
- World Health Organization 2009Influenza (seasonal) fact sheet. http://www.who.int/mediacentre/factsheets/fs211/en/
-
- Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–1169. - PubMed
-
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010. p. CD004876. - PubMed
-
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. - PubMed
-
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007;7:658–666. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

