Role of telomere dysfunction in cardiac failure in Duchenne muscular dystrophy
- PMID: 23831727
- PMCID: PMC3774175
- DOI: 10.1038/ncb2790
Role of telomere dysfunction in cardiac failure in Duchenne muscular dystrophy
Abstract
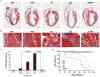
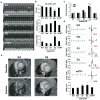
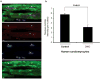
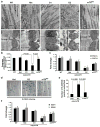
Duchenne muscular dystrophy (DMD), the most common inherited muscular dystrophy of childhood, leads to death due to cardiorespiratory failure. Paradoxically, mdx mice with the same genetic deficiency of dystrophin exhibit minimal cardiac dysfunction, impeding the development of therapies. We postulated that the difference between mdx and DMD might result from differences in telomere lengths in mice and humans. We show here that, like DMD patients, mice that lack dystrophin and have shortened telomeres (mdx/mTR(KO)) develop severe functional cardiac deficits including ventricular dilation, contractile and conductance dysfunction, and accelerated mortality. These cardiac defects are accompanied by telomere erosion, mitochondrial fragmentation and increased oxidative stress. Treatment with antioxidants significantly retards the onset of cardiac dysfunction and death of mdx/mTR(KO) mice. In corroboration, all four of the DMD patients analysed had 45% shorter telomeres in their cardiomyocytes relative to age- and sex-matched controls. We propose that the demands of contraction in the absence of dystrophin coupled with increased oxidative stress conspire to accelerate telomere erosion culminating in cardiac failure and death. These findings provide strong support for a link between telomere length and dystrophin deficiency in the etiology of dilated cardiomyopathy in DMD and suggest preventive interventions.
Conflict of interest statement
The authors declare no competing financial interests
Figures


References
-
- Liew CC, Dzau VJ. Molecular genetics and genomics of heart failure. Nat Rev Genet. 2004;5:811–825. - PubMed
-
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. - PubMed
-
- Bushby K, Muntoni F, Bourke JP. 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy. 7th–9th June 2002, Naarden, the Netherlands. Neuromuscul Disord. 2003;13:166–172. - PubMed
-
- Baxter P. Treatment of the heart in Duchenne muscular dystrophy. Dev Med Child Neurol. 2006;48:163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 AG009521/AG/NIA NIH HHS/United States
- NS069375/NS/NINDS NIH HHS/United States
- P30 NS069375/NS/NINDS NIH HHS/United States
- AG020961/AG/NIA NIH HHS/United States
- R21 AG044815/AG/NIA NIH HHS/United States
- R01 AG020961/AG/NIA NIH HHS/United States
- R01 HL096113/HL/NHLBI NIH HHS/United States
- HL100397/HL/NHLBI NIH HHS/United States
- HL096113/HL/NHLBI NIH HHS/United States
- HL061535/HL/NHLBI NIH HHS/United States
- R01CA84628/CA/NCI NIH HHS/United States
- U01 HL100397/HL/NHLBI NIH HHS/United States
- P50 CA058236/CA/NCI NIH HHS/United States
- AG009521/AG/NIA NIH HHS/United States
- R01 HL061535/HL/NHLBI NIH HHS/United States
- P50CA058236/CA/NCI NIH HHS/United States
- R01 AG009521/AG/NIA NIH HHS/United States
- R01 CA084628/CA/NCI NIH HHS/United States
- P30 AR057220/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

