Spiral countercurrent chromatography
- PMID: 23833207
- PMCID: PMC3702229
- DOI: 10.1093/chromsci/bmt058
Spiral countercurrent chromatography
Erratum in
- J Chromatogr Sci. 2013 Oct;51(9):891
Abstract
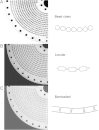
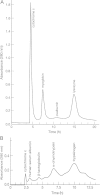
For many years, high-speed countercurrent chromatography conducted in open tubing coils has been widely used for the separation of natural and synthetic compounds. In this method, the retention of the stationary phase is solely provided by the Archimedean screw effect by rotating the coiled column in the centrifugal force field. However, the system fails to retain enough of the stationary phase for polar solvent systems such as the aqueous-aqueous polymer phase systems. To address this problem, the geometry of the coiled channel was modified to a spiral configuration so that the system could utilize the radially acting centrifugal force. This successfully improved the retention of the stationary phase. Two different types of spiral columns were fabricated: the spiral disk assembly, made by stacking multiple plastic disks with single or four interwoven spiral channels connected in series, and the spiral tube assembly, made by inserting the tetrafluoroethylene tubing into a spiral frame (spiral tube support). The capabilities of these column assemblies were successfully demonstrated by separations of peptides and proteins with polar two-phase solvent systems whose stationary phases had not been well retained in the earlier multilayer coil separation column for high-speed countercurrent chromatography.
Figures











References
-
- Ito Y., Bowman R.L. Countercurrent chromatography: Liquid-liquid partition chromatography without solid support. Science. 1970;167:281–283. - PubMed
-
- Ito Y., Bowman R.L. Countercurrent chromatography: Liquid-liquid partition chromatography without solid support. Journal of Chromatographic Science. 1970;8:315–323. - PubMed
-
- Ito Y., Bowman R.L. Countercurrent chromatography. Analytical Chemistry. 1971;43 69A.
-
- Ito Y., Bowman R.L. Countercurrent chromatography with the flow-through coil planet centrifuge. Journal of Chromatographic Science. 1973;11:284–291. - PubMed
-
- Ito Y. New York, NY: Marcel Dekker, Inc; 1988. Principle and instrumentation of countercurrent chromatography. In Countercurrent chromatography: Theory and practice, Chapter 3; pp. 79–492.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

