Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells
- PMID: 23833237
- PMCID: PMC3884521
- DOI: 10.4049/jimmunol.1300658
Butyrophilin 3A1 plays an essential role in prenyl pyrophosphate stimulation of human Vγ2Vδ2 T cells
Abstract
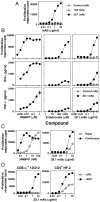
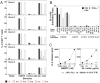
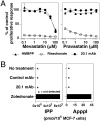
Most human γδ T cells express Vγ2Vδ2 TCRs and play important roles in microbial and tumor immunity. Vγ2Vδ2 T cells are stimulated by self- and foreign prenyl pyrophosphate intermediates in isoprenoid synthesis. However, little is known about the molecular basis for this stimulation. We find that a mAb specific for butyrophilin 3 (BTN3)/CD277 Ig superfamily proteins mimics prenyl pyrophosphates. The 20.1 mAb stimulated Vγ2Vδ2 T cell clones regardless of their functional phenotype or developmental origin and selectively expanded blood Vγ2Vδ2 T cells. The γδ TCR mediates 20.1 mAb stimulation because IL-2 is released by β(-) Jurkat cells transfected with Vγ2Vδ2 TCRs. 20.1 stimulation was not due to isopentenyl pyrophosphate (IPP) accumulation because 20.1 treatment of APC did not increase IPP levels. In addition, stimulation was not inhibited by statin treatment, which blocks IPP production. Importantly, small interfering RNA knockdown of BTN3A1 abolished stimulation by IPP that could be restored by re-expression of BTN3A1 but not by BTN3A2 or BTN3A3. Rhesus monkey and baboon APC presented HMBPP and 20.1 to human Vγ2Vδ2 T cells despite amino acid differences in BTN3A1 that localize to its outer surface. This suggests that the conserved inner and/or top surfaces of BTN3A1 interact with its counterreceptor. Although no binding site exists on the BTN3A1 extracellular domains, a model of the intracellular B30.2 domain predicts a basic pocket on its binding surface. However, BTN3A1 did not preferentially bind a photoaffinity prenyl pyrophosphate. Thus, BTN3A1 is required for stimulation by prenyl pyrophosphates but does not bind the intermediates with high affinity.
Figures





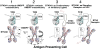
Similar articles
-
Sensor Function for Butyrophilin 3A1 in Prenyl Pyrophosphate Stimulation of Human Vγ2Vδ2 T Cells.J Immunol. 2015 Nov 15;195(10):4583-94. doi: 10.4049/jimmunol.1500314. Epub 2015 Oct 16. J Immunol. 2015. PMID: 26475929 Free PMC article.
-
Critical Roles for Coiled-Coil Dimers of Butyrophilin 3A1 in the Sensing of Prenyl Pyrophosphates by Human Vγ2Vδ2 T Cells.J Immunol. 2019 Aug 1;203(3):607-626. doi: 10.4049/jimmunol.1801252. Epub 2019 Jun 21. J Immunol. 2019. PMID: 31227581 Free PMC article.
-
Activation of human γδ T cells by cytosolic interactions of BTN3A1 with soluble phosphoantigens and the cytoskeletal adaptor periplakin.J Immunol. 2015 Mar 1;194(5):2390-8. doi: 10.4049/jimmunol.1401064. Epub 2015 Jan 30. J Immunol. 2015. PMID: 25637025 Free PMC article.
-
Protective immune responses of major Vγ2Vδ2 T-cell subset in M. tuberculosis infection.Curr Opin Immunol. 2016 Oct;42:105-112. doi: 10.1016/j.coi.2016.06.005. Epub 2016 Aug 1. Curr Opin Immunol. 2016. PMID: 27491008 Free PMC article. Review.
-
ABCA1, apoA-I, and BTN3A1: A Legitimate Ménage à Trois in Dendritic Cells.Front Immunol. 2018 Jun 8;9:1246. doi: 10.3389/fimmu.2018.01246. eCollection 2018. Front Immunol. 2018. PMID: 29937767 Free PMC article. Review.
Cited by
-
Gamma delta T-cell reconstitution after allogeneic HCT: A platform for cell therapy.Front Immunol. 2022 Aug 29;13:971709. doi: 10.3389/fimmu.2022.971709. eCollection 2022. Front Immunol. 2022. PMID: 36105821 Free PMC article. Review.
-
HMBPP Analog Prodrugs Bypass Energy-Dependent Uptake To Promote Efficient BTN3A1-Mediated Malignant Cell Lysis by Vγ9Vδ2 T Lymphocyte Effectors.J Immunol. 2016 Jul 15;197(2):419-28. doi: 10.4049/jimmunol.1501833. Epub 2016 Jun 6. J Immunol. 2016. PMID: 27271567 Free PMC article.
-
γδ T Cells in HIV Disease: Past, Present, and Future.Front Immunol. 2015 Jan 30;5:687. doi: 10.3389/fimmu.2014.00687. eCollection 2014. Front Immunol. 2015. PMID: 25688241 Free PMC article. Review.
-
BTN3A molecules considerably improve Vγ9Vδ2T cells-based immunotherapy in acute myeloid leukemia.Oncoimmunology. 2016 Apr 25;5(10):e1146843. doi: 10.1080/2162402X.2016.1146843. eCollection 2016. Oncoimmunology. 2016. PMID: 27853633 Free PMC article.
-
Anti-Tumor Activity and Immunotherapeutic Potential of a Bisphosphonate Prodrug.Sci Rep. 2017 Jul 20;7(1):5987. doi: 10.1038/s41598-017-05553-0. Sci Rep. 2017. PMID: 28729550 Free PMC article.
References
-
- Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vγ2Vδ2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. - PubMed
-
- Hintz M, Reichenberg A, Altincicek B, Bahr U, Gschwind RM, Kollas AK, Beck E, Wiesner J, Eberl M, Jomaa H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells in Escherichia coli. FEBS Lett. 2001;509:317–322. - PubMed
-
- Puan KJ, Jin C, Wang H, Sarikonda G, Raker AM, Lee HK, Samuelson MI, Märker-Hermann E, Pasa-Tolic L, Nieves E, Giner JL, Kuzuyama T, Morita CT. Preferential recognition of a microbial metabolite by human Vγ2Vδ2 T cells. Int Immunol. 2007;19:657–673. - PubMed
-
- Kunzmann V, Bauer E, Wilhelm M. γ/δ T-cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

