Synthesis and biological evaluation of di- and tri-substituted imidazoles as safer anti-inflammatory-antifungal agents
- PMID: 23833522
- PMCID: PMC3697195
- DOI: 10.4103/0975-7406.111822
Synthesis and biological evaluation of di- and tri-substituted imidazoles as safer anti-inflammatory-antifungal agents
Abstract
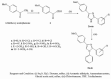
Purpose: In view of the potential pharmacophoric nature of imidazole nucleus, two series of imidazole derivatives, 2,4-disubstituted-1 H-imidazoles (2a-m) and 1,2,4-trisubstituted-1 H-imidazoles (3a-m), were synthesized with an aim of obtaining dual acting compounds i.e., anti-inflammatory and antifungal agents.
Materials and methods: The title compounds were synthesized from 4-methoxyphenyl glyoxal (1) following multistep synthesis, and their structures were established on the basis of modern analytical techniques (IR, NMR and MS). The synthesized imidazoles were tested for their in vivo anti-inflammatory activity. In addition to that, some compounds were also evaluated for their analgesic and ulcerogenic effects. The compounds were also evaluated for their in vitro antifungal activity.
Results: Di- and tri-substituted imidazole derivatives (2a-m and 3a-m) were successfully synthesized. In in vivo anti-inflammatory test, six compounds (2 h, 2 l, 3 g, 3 h, 3 l and 3 m) exhibited good anti-inflammatory activity (49.58 to 58.02% inhibition) with minimal GI irritation (severity index; 0.17 to 0.34). These compounds were also tested for their analgesic activity and showed appreciable protection (40.53 to 49.60% protection) against saline-induced writhing test. Indomethacin was used as standard drug for comparison. In antifungal test, two compounds (3 h and 3 l) displayed appreciable antifungal activity (MIC; 12.5 μg mL(-1)) against the fungal strains tested.
Conclusion: Two compounds, 2-(4-nitrophenyl)-4-(4-methoxyphenyl)-1-phenyl-1H-imidazole (3 h) and 2,4-di-(4-methoxyphenyl)-1-phenyl-1H-imidazole (3 l), emerged as lead compounds having dual biological activities; good anti-inflammatory as well as antifungal effect with lesser GI irritation.
Keywords: Analgesic; anti-inflammatory; antifungal; imidazole; ulcerogenicity.
Conflict of interest statement
Figures
References
-
- Bekhit AA, Aziem TA. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg Med Chem. 2004;12:1935–45. - PubMed
-
- Cioli V, Putzolu S, Rossi V, Sorza Barcellona P, Corradino C. The role of direct tissue contact in the production of gastro-intestinal ulcer by anti-inflammatory drugs in rats. Toxicol Appl Pharmacol. 1979;50:283–9. - PubMed
-
- Puratchikody A, Doble M. Antinociceptive and anti-inflammatory activities and QSAR studies on 2-substituted-4,5-diphenyl-1 H-imidazoles. Bioorg Med Chem. 2007;15:1083–90. - PubMed
-
- Frank PV, Girish KS, Kalluraya B. Solvent-free microwave-assisted synthesis of oxadiazoles containing imidazole moiety. J Chem Sci. 2007;119:41–6.
-
- Ramachandran R, Rani M, Senthan S, Jeong YT, Kabilan S. Synthesis, spectral, crystal structure and in vitro antimicrobial evaluation of imidazole/benzotriazole substituted piperidin-4-one derivatives. Eur J Med Chem. 2011;46:1926–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources