Incidence and risk of hypomagnesemia in advanced cancer patients treated with cetuximab: A meta-analysis
- PMID: 23833666
- PMCID: PMC3700916
- DOI: 10.3892/ol.2013.1301
Incidence and risk of hypomagnesemia in advanced cancer patients treated with cetuximab: A meta-analysis
Abstract
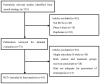
Hypomagnesemia is a serious adverse event for patients treated with cetuximab, an inhibitor of endothelial growth factor receptor (EGFR). However, no significant association has yet been established between cetuximab and hypomagnesemia in randomized controlled clinical trials (RCTs). The present study conducted a systematic review and meta-analysis of published RCTs to assess the overall risk of hypomagnesemia associated with cetuximab. PubMed, the Cochrane Central Register of Controlled Trials, Embase and the American Society of Clinical Oncology conferences were searched for relevant RCTs. Quantitative analysis was carried out to evaluate the association between hypomagnesemia and cetuximab. A total of 7,045 patients with a variety of advanced cancers from 10 trials were included in the analysis. The overall incidence of grade 3/4 hypomagnesemia in patients receiving cetuximab was 3.9% [95% confidence interval (CI), 2.6-4.3%]. Patients treated with cetuximab had a significantly increased risk of grade 3/4 hypomagnesemia compared with patients treated with control medication, with a relative risk (RR) of 8.60 (95% CI, 5.08-14.54). Risk was observed to vary with tumor type. The study concluded that cetuximab is associated with a significant risk of hypomagnesemia in patients with advanced cancer receiving concurrent chemotherapy.
Keywords: advanced cancer; cetuximab; hypomagnesemia; meta-analysis.
Figures

Similar articles
-
Incidence and risk of severe neutropenia in advanced cancer patients treated with cetuximab: a meta-analysis.Drugs R D. 2011 Dec 1;11(4):317-26. doi: 10.2165/11598190-000000000-00000. Drugs R D. 2011. PMID: 22133387 Free PMC article.
-
Meta-analysis of incidence and risk of hypomagnesemia with cetuximab for advanced cancer.Chemotherapy. 2010;56(6):459-65. doi: 10.1159/000321011. Epub 2010 Nov 18. Chemotherapy. 2010. PMID: 21088398
-
Electrolyte disorders assessment in solid tumor patients treated with anti-EGFR monoclonal antibodies: a pooled analysis of 25 randomized clinical trials.Tumour Biol. 2015 May;36(5):3471-82. doi: 10.1007/s13277-014-2983-9. Epub 2014 Dec 28. Tumour Biol. 2015. PMID: 25542231 Free PMC article.
-
Hematologic toxicity assessment in solid tumor patients treated with cetuximab: a pooled analysis of 18 randomized controlled trials.Int J Cancer. 2015 Feb 15;136(4):936-44. doi: 10.1002/ijc.29045. Epub 2014 Jul 8. Int J Cancer. 2015. PMID: 24975040
-
Infectious complications in cancer patients treated with anti-EGFR monoclonal antibodies cetuximab and panitumumab: a systematic review and meta-analysis.Cancer Treat Rev. 2014 Dec;40(10):1221-9. doi: 10.1016/j.ctrv.2014.09.002. Epub 2014 Sep 19. Cancer Treat Rev. 2014. PMID: 25288497
Cited by
-
Refractory hypokalemia caused by cetuximab with advanced colorectal cancer patients: the case series and literature review.Anticancer Drugs. 2022 Jan 1;33(1):e789-e794. doi: 10.1097/CAD.0000000000001212. Anticancer Drugs. 2022. PMID: 34419963 Free PMC article.
-
Magnesium Absorption in Intestinal Cells: Evidence of Cross-Talk between EGF and TRPM6 and Novel Implications for Cetuximab Therapy.Nutrients. 2020 Oct 26;12(11):3277. doi: 10.3390/nu12113277. Nutrients. 2020. PMID: 33114586 Free PMC article.
-
Safety and Immunogenicity of a Human Epidermal Growth Factor Receptor 1 (HER1)-Based Vaccine in Prostate Castration-Resistant Carcinoma Patients: A Dose-Escalation Phase I Study Trial.Front Pharmacol. 2017 May 10;8:263. doi: 10.3389/fphar.2017.00263. eCollection 2017. Front Pharmacol. 2017. PMID: 28539888 Free PMC article.
-
A Comprehensive Review of Clinical Trials on EGFR Inhibitors Such as Cetuximab and Panitumumab as Monotherapy and in Combination for Treatment of Metastatic Colorectal Cancer.Avicenna J Med Biotechnol. 2015 Oct-Dec;7(4):134-44. Avicenna J Med Biotechnol. 2015. PMID: 26605007 Free PMC article. Review.
-
Research progress on common adverse events caused by targeted therapy for colorectal cancer.Oncol Lett. 2018 Jul;16(1):27-33. doi: 10.3892/ol.2018.8651. Epub 2018 May 7. Oncol Lett. 2018. PMID: 29928383 Free PMC article. Review.
References
-
- Brandes AA, Franceschi E, Tosoni A, Hegi ME, Stupp R. Epidermal growth factor receptor inhibitors in neuro-oncology: hopes and disappointments. Clin Cancer Res. 2008;14:957–960. - PubMed
-
- Reynolds NA, Wagstaff AJ. Cetuximab: in the treatment of metastatic colorectal cancer. Drugs. 2004;64:109–121. - PubMed
-
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, Chau I, Van Cutsem E. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
