A Single Gradient Stability-Indicating Reversed-Phase LC Method for the Estimation of Impurities in Omeprazole and Domperidone Capsules
- PMID: 23833712
- PMCID: PMC3700074
- DOI: 10.3797/scipharm.1209-12
A Single Gradient Stability-Indicating Reversed-Phase LC Method for the Estimation of Impurities in Omeprazole and Domperidone Capsules
Abstract
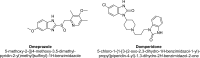
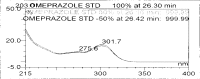
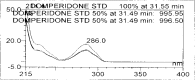
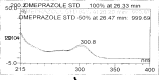
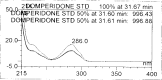
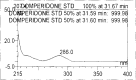
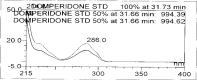
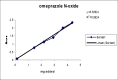
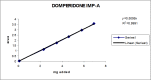
A gradient reversed-phase liquid chromatographic (RP-LC) method was developed for the quantitative estimation of impurities in the pharmaceutical dosage form of Omeprazole and Domperidone capsules. The developed method is a stability-indicating test method for the estimation of impurities generated during the formulation and storage of Omeprazole and Domperidone capsules. The chromatographic separation was achieved on a column packed with octadecyl silane, having a column length of 250 mm and diameter of 4.6 mm with a particle size of 5 μm, and by following a gradient program using a combination of a monobasic potassium phosphate buffer (0.05M) and acetonitrile. Since the spectral properties were similar, both compounds' individual impurities were estimated at 285 nm. Forced degradation studies were performed on Omeprazole pellets (enteric coated) and Domperidone pellets (SR coated) encapsulated in size '1' hard gelatin capsules. Omeprazole and Domperidone were degraded using acid hydrolysis (0.1 N hydrochloric acid), base (0.1 N sodium hydroxide), oxidation (50% hydrogen peroxide), heat (105 °C), and UV light (254 nm). The established method was validated and found to be linear, accurate, precise, specific, robust, and rugged.
Keywords: Domperidone; Forced Degradation; Method validation; Omeprazole; RP-LC.
Figures






















Similar articles
-
Complexity in estimation of esomeprazole and its related impurities' stability in various stress conditions in low-dose aspirin and esomeprazole magnesium capsules.Sci Pharm. 2013 Apr-Jun;81(2):475-92. doi: 10.3797/scipharm.1212-13. Epub 2013 Feb 18. Sci Pharm. 2013. PMID: 23833714 Free PMC article.
-
Stability-indicating LC-UV method for the determination of eszopiclone and degradation impurities in tablet dosage form.J Chromatogr Sci. 2014 Apr;52(4):293-7. doi: 10.1093/chromsci/bmt027. Epub 2013 Apr 3. J Chromatogr Sci. 2014. PMID: 23552846
-
Forced degradation study and characterization of main impurities of ibuprofen soft gelatin capsules by LC-MS-QTOF.Pharmazie. 2021 Apr 1;76(4):138-144. doi: 10.1691/ph.2021.0126. Pharmazie. 2021. PMID: 33849697
-
Development and Validation of a Precise, Single HPLC Method for the Determination of Tolperisone Impurities in API and Pharmaceutical Dosage Forms.Sci Pharm. 2013 Jan-Mar;81(1):123-38. doi: 10.3797/scipharm.1209-17. Epub 2012 Nov 5. Sci Pharm. 2013. PMID: 23641333 Free PMC article.
-
Development of RP UPLC-TOF/MS, stability indicating method for omeprazole and its related substances by applying two level factorial design; and identification and synthesis of non-pharmacopoeial impurities.J Pharm Biomed Anal. 2016 Jan 25;118:370-379. doi: 10.1016/j.jpba.2015.10.005. Epub 2015 Nov 21. J Pharm Biomed Anal. 2016. PMID: 26600119
Cited by
-
Development and Validation of a Novel Stability-Indicating RP-HPLC Method for the Simultaneous Determination of Related Substances of Ketoprofen and Omeprazole in Combined Capsule Dosage Form.J Chromatogr Sci. 2016 May-Jun;54(5):765-75. doi: 10.1093/chromsci/bmw008. Epub 2016 Feb 8. J Chromatogr Sci. 2016. PMID: 26860397 Free PMC article.
-
Design of Experiment (DOE) Utilization to Develop a Simple and Robust Reversed-Phase HPLC Technique for Related Substances' Estimation of Omeprazole Formulations.Sci Pharm. 2013 Aug 12;81(4):1043-56. doi: 10.3797/scipharm.1306-06. Print 2013 Oct-Dec. Sci Pharm. 2013. PMID: 24482772 Free PMC article.
References
-
- Aydan Y, Rustii O. Specific H+/K+-ATPase inhibitors decreased contractile responses of isolated rat vas deferens. Pharmacol Res. 2006;54:397–405. http://dx.doi.org/10.1016/j.phrs.2006.07.005. - DOI - PubMed
-
- Shimatani T, Inoue M, Kuroiwa T, Xu J, Mieno H, Tazuma S. Acid suppressive effect of Generic Omeprazole, comparison of 3 brands of Generics with Omeprazole. Dig Liver Dis. 2006;38:554–559. http://dx.doi.org/10.1016/j.dld.2006.01.032. - DOI - PubMed
-
- Ahmad N, Keith-Ferris J, Gooden E, Abell T. Making a case for Domperidone in the treatment of gastro intestinal motility disorders. Curr Opin Pharmacol. 2006;6:571–576. http://dx.doi.org/10.1016/j.coph.2006.07.004. - DOI - PubMed
-
- Drug Bank Mechanism of action of Domperidone. Domperidone; Accession Number: APRD00418.
-
- Naser LR, Keven CB, Angela DMK. A simple sensitive bioanalytical assay for simultaneous determination of omeprazole and its 3 major metabolites in human blood plasma using RP-HPLC after a simple liquid-liquid extraction procedure. J Chromatogr B. 2006;844:314–321. http://dx.doi.org/10.1016/j.jchromb.2006.07.047. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
