Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors
- PMID: 23834311
- PMCID: PMC3788085
- DOI: 10.1111/cmi.12164
Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors
Abstract
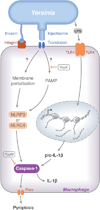
The innate immune system of mammals responds to microbial infection through detection of conserved molecular determinants called 'pathogen-associated molecular patterns' (PAMPs). Pathogens use virulence factors to counteract PAMP-directed responses. The innate immune system can in turn recognize signals generated by virulence factors, allowing for a heightened response to dangerous pathogens. Many Gram-negative bacterial pathogens encode type III secretion systems (T3SSs) that translocate effector proteins, subvert PAMP-directed responses and are critical for infection. A plasmid-encoded T3SS in the human-pathogenic Yersinia species translocates seven effectors into infected host cells. Delivery of effectors by the T3SS requires plasma membrane insertion of two translocators, which are thought to form a channel called a translocon. Studies of the Yersinia T3SS have provided key advances in our understanding of how innate immune responses are generated by perturbations in plasma membrane and other signals that result from translocon insertion. Additionally, studies in this system revealed that effectors function to inhibit innateimmune responses resulting from insertion of translocons into plasma membrane. Here, we review these advances with the goal of providing insight into how a T3SS can activate and inhibit innate immune responses, allowing a virulent pathogen to bypass host defences.
© 2013 John Wiley & Sons Ltd.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

