Transient lymphopenia breaks costimulatory blockade-based peripheral tolerance and initiates cardiac allograft rejection
- PMID: 23834725
- PMCID: PMC4216721
- DOI: 10.1111/ajt.12342
Transient lymphopenia breaks costimulatory blockade-based peripheral tolerance and initiates cardiac allograft rejection
Abstract
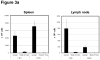
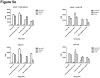
Lymphopenia is induced by lymphoablative therapies and chronic viral infections. We assessed the impact of lymphopenia on cardiac allograft survival in recipients conditioned with peritransplant costimulatory blockade (CB) to promote long-term graft acceptance. After vascularized MHC-mismatched heterotopic heart grafts were stably accepted through CB, lymphopenia was induced on day 60 posttransplant by 6.5 Gy irradiation or by administration of anti-CD4 plus anti-CD8 mAb. Long-term surviving allografts were gradually rejected after lymphodepletion (MST = 74 ± 5 days postirradiation). Histological analyses indicated signs of severe rejection in allografts following lymphodepletion, including mononuclear cell infiltration and obliterative vasculopathy. Lymphodepletion of CB conditioned recipients induced increases in CD44(high) effector/memory T cells in lymphatic organs and strong recovery of donor-reactive T cell responses, indicating lymphopenia-induced proliferation (LIP) and donor alloimmune responses occurring in the host. T regulatory (CD4(+) Foxp(3+)) cell and B cell numbers as well as donor-specific antibody titers also increased during allograft rejection in CB conditioned recipients given lymphodepletion. These observations suggest that allograft rejection following partial lymphocyte depletion is mediated by LIP of donor-reactive memory T cells. As lymphopenia may cause unexpected rejection of stable allografts, adequate strategies must be developed to control T cell proliferation and differentiation during lymphopenia.
Keywords: Costimulatory blockade; lymphopenia-induced proliferation; memory T cell.
© Copyright 2013 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
The authors have no financial conflict of interest to declare.
Figures















Similar articles
-
NK cells are required for costimulatory blockade induced tolerance to vascularized allografts.Transplantation. 2012 Sep 27;94(6):575-84. doi: 10.1097/TP.0b013e318264d3c4. Transplantation. 2012. PMID: 22914174 Free PMC article.
-
Antibody-mediated rejection of cardiac allografts in CCR5-deficient recipients.J Immunol. 2007 Oct 15;179(8):5238-45. doi: 10.4049/jimmunol.179.8.5238. J Immunol. 2007. PMID: 17911609
-
Relative Frequencies of Alloantigen-Specific Helper CD4 T Cells and B Cells Determine Mode of Antibody-Mediated Allograft Rejection.Front Immunol. 2019 Jan 22;9:3039. doi: 10.3389/fimmu.2018.03039. eCollection 2018. Front Immunol. 2019. PMID: 30740108 Free PMC article.
-
The Role of TNFR2 and DR3 in the In Vivo Expansion of Tregs in T Cell Depleting Transplantation Regimens.Int J Mol Sci. 2020 May 9;21(9):3347. doi: 10.3390/ijms21093347. Int J Mol Sci. 2020. PMID: 32397343 Free PMC article. Review.
-
Endogenous memory T cells with donor-reactivity: early post-transplant mediators of acute graft injury in unsensitized recipients.Transpl Int. 2021 Aug;34(8):1360-1373. doi: 10.1111/tri.13900. Epub 2021 Jun 29. Transpl Int. 2021. PMID: 33963616 Free PMC article. Review.
Cited by
-
Depletion-Resistant CD4 T Cells Enhance Thymopoiesis During Lymphopenia.Am J Transplant. 2017 Aug;17(8):2008-2019. doi: 10.1111/ajt.14309. Epub 2017 May 17. Am J Transplant. 2017. PMID: 28397358 Free PMC article.
-
Prevalence and pathogenicity of autoantibodies in patients with idiopathic CD4 lymphopenia.J Clin Invest. 2020 Oct 1;130(10):5326-5337. doi: 10.1172/JCI136254. J Clin Invest. 2020. PMID: 32634122 Free PMC article.
-
Challenges and opportunities in targeting the CD28/CTLA-4 pathway in transplantation and autoimmunity.Expert Opin Biol Ther. 2017 Aug;17(8):1001-1012. doi: 10.1080/14712598.2017.1333595. Epub 2017 May 30. Expert Opin Biol Ther. 2017. PMID: 28525959 Free PMC article. Review.
-
Recollective homeostasis and the immune consequences of peritransplant depletional induction therapy.Immunol Rev. 2014 Mar;258(1):167-82. doi: 10.1111/imr.12155. Immunol Rev. 2014. PMID: 24517433 Free PMC article. Review.
-
Tracking of TCR-Transgenic T Cells Reveals That Multiple Mechanisms Maintain Cardiac Transplant Tolerance in Mice.Am J Transplant. 2016 Oct;16(10):2854-2864. doi: 10.1111/ajt.13814. Epub 2016 May 5. Am J Transplant. 2016. PMID: 27091509 Free PMC article.
References
-
- Baczkowska T, Durlik M. Calcineurin inhibitor sparing immunosuppressive regimens in kidney allograft recipients. Pol Arch Med Wewn. 2009 May;119(5):318–25. - PubMed
-
- Vathsala A. Preventing renal transplant failure. Ann Acad Med Singapore. 2005 Jan;34(1):36–43. - PubMed
-
- Boisgerault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol. 2001 Aug 15;167(4):1891–9. - PubMed
-
- Billing H, Rieger S, Ovens J, Susal C, Melk A, Waldherr R, et al. Successful treatment of chronic antibody-mediated rejection with IVIG and rituximab in pediatric renal transplant recipients. Transplantation. 2008 Nov 15;86(9):1214–21. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

