Structure-function relationships in hydrophobins: probing the role of charged side chains
- PMID: 23835172
- PMCID: PMC3754166
- DOI: 10.1128/AEM.01493-13
Structure-function relationships in hydrophobins: probing the role of charged side chains
Abstract
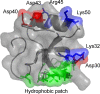
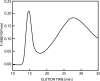
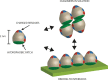
Hydrophobins are small fungal proteins that are amphiphilic and have a strong tendency to assemble at interfaces. By taking advantage of this property, hydrophobins have been used for a number of applications: as affinity tags in protein purification, for protein immobilization, such as in foam stabilizers, and as dispersion agents for insoluble drug molecules. Here, we used site-directed mutagenesis to gain an understanding of the molecular basis of their properties. We especially focused on the role of charged amino acids in the structure of hydrophobins. For this purpose, fusion proteins consisting of Trichoderma reesei hydrophobin I (HFBI) and the green fluorescent protein (GFP) that contained various combinations of substitutions of charged amino acids (D30, K32, D40, D43, R45, K50) in the HFBI structure were produced. The effects of the introduced mutations on binding, oligomerization, and partitioning were characterized in an aqueous two-phase system. It was found that some substitutions caused better surface binding and reduced oligomerization, while some showed the opposite effects. However, all mutations decreased partitioning in surfactant systems, indicating that the different functions are not directly correlated and that partitioning is dependent on finely tuned properties of hydrophobins. This work shows that not all functions in self-assembly are connected in a predictable way and that a simple surfactant model for hydrophobin function is insufficient.
Figures


Similar articles
-
Novel Hydrophobin Fusion Tags for Plant-Produced Fusion Proteins.PLoS One. 2016 Oct 5;11(10):e0164032. doi: 10.1371/journal.pone.0164032. eCollection 2016. PLoS One. 2016. PMID: 27706254 Free PMC article.
-
Behavior of Trichoderma reesei hydrophobins in solution: interactions, dynamics, and multimer formation.Biochemistry. 2006 Jul 18;45(28):8590-8. doi: 10.1021/bi060620y. Biochemistry. 2006. PMID: 16834333
-
Surface adhesion of fusion proteins containing the hydrophobins HFBI and HFBII from Trichoderma reesei.Protein Sci. 2002 Sep;11(9):2257-66. doi: 10.1110/ps.0207902. Protein Sci. 2002. PMID: 12192081 Free PMC article.
-
Hydrophobins: the protein-amphiphiles of filamentous fungi.FEMS Microbiol Rev. 2005 Nov;29(5):877-96. doi: 10.1016/j.femsre.2005.01.004. Epub 2005 Feb 21. FEMS Microbiol Rev. 2005. PMID: 16219510 Review.
-
Fungal Hydrophobins and Their Self-Assembly into Functional Nanomaterials.Adv Exp Med Biol. 2019;1174:161-185. doi: 10.1007/978-981-13-9791-2_5. Adv Exp Med Biol. 2019. PMID: 31713199 Review.
Cited by
-
Novel Hydrophobin Fusion Tags for Plant-Produced Fusion Proteins.PLoS One. 2016 Oct 5;11(10):e0164032. doi: 10.1371/journal.pone.0164032. eCollection 2016. PLoS One. 2016. PMID: 27706254 Free PMC article.
-
Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability.Nanomaterials (Basel). 2014 Sep 17;4(3):827-843. doi: 10.3390/nano4030827. Nanomaterials (Basel). 2014. PMID: 28344251 Free PMC article.
-
Comparative genomics and expression levels of hydrophobins from eight mycorrhizal genomes.Mycorrhiza. 2017 May;27(4):383-396. doi: 10.1007/s00572-016-0758-4. Epub 2017 Jan 9. Mycorrhiza. 2017. PMID: 28066872
-
The self-assembly of monosubstituted BODIPY and HFBI-RGD.RSC Adv. 2018 Jun 12;8(38):21472-21479. doi: 10.1039/c8ra03687j. eCollection 2018 Jun 8. RSC Adv. 2018. PMID: 35539954 Free PMC article.
-
Modular architecture of protein binding units for designing properties of cellulose nanomaterials.Angew Chem Int Ed Engl. 2015 Oct 5;54(41):12025-8. doi: 10.1002/anie.201505980. Epub 2015 Aug 25. Angew Chem Int Ed Engl. 2015. PMID: 26305491 Free PMC article.
References
-
- Linder MB, Szilvay GR, Nakari-Setälä T, Penttilä ME. 2005. Hydrophobins: the protein-amphiphiles of filamentous fungi. FEMS Microbiol. Rev. 29:877–896 - PubMed
-
- Wösten HAB, Willey JM. 2000. Surface-active proteins enable microbial aerial hyphae to grow into the air. Microbiology 146:767–773 - PubMed
-
- Cox PW, Hooley P. 2009. Hydrophobins: new prospects for biotechnology. Fungal Biol. Rev. 23:40–47
-
- Cox AR, Aldred DL, Russell AB. 2009. Exceptional stability of food foams using class II hydrophobin HFBII. Food Hydrocolloids 23:366–376
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

