Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders
- PMID: 23835333
- PMCID: PMC3806618
- DOI: 10.2337/db13-0345
Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders
Abstract
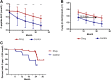
Trials of immune therapies in new-onset type 1 diabetes (T1D) have shown success, but not all subjects respond, and the duration of response is limited. Our aim was to determine whether two courses of teplizumab, an Fc receptor-nonbinding anti-CD3 monoclonal antibody, reduces the decline in C-peptide levels in patients with T1D 2 years after disease onset. We also set out to identify characteristics of responders. We treated 52 subjects with new-onset T1D with teplizumab for 2 weeks at diagnosis and after 1 year in an open-label, randomized, controlled trial. In the intent to treat analysis of the primary end point, patients treated with teplizumab had a reduced decline in C-peptide at 2 years (mean -0.28 nmol/L [95% CI -0.36 to -0.20]) versus control (mean -0.46 nmol/L [95% CI -0.57 to -0.35]; P = 0.002), a 75% improvement. The most common adverse events were rash, transient upper respiratory infections, headache, and nausea. In a post hoc analysis we characterized clinical responders and found that metabolic (HbA1c and insulin use) and immunologic features distinguished this group from those who did not respond to teplizumab. We conclude that teplizumab treatment preserves insulin production and reduces the use of exogenous insulin in some patients with new-onset T1D. Metabolic and immunologic features at baseline can identify a subgroup with robust responses to immune therapy.
Trial registration: ClinicalTrials.gov NCT00129259.
Figures




Comment in
-
The compelling case for anti-CD3 in type 1 diabetes.Diabetes. 2013 Nov;62(11):3656-7. doi: 10.2337/db13-1157. Diabetes. 2013. PMID: 24158991 Free PMC article. No abstract available.
References
-
- Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 - PubMed
-
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

