Skeletal muscle triacylglycerol hydrolysis does not influence metabolic complications of obesity
- PMID: 23835334
- PMCID: PMC3781480
- DOI: 10.2337/db13-0500
Skeletal muscle triacylglycerol hydrolysis does not influence metabolic complications of obesity
Abstract
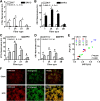
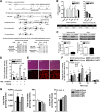
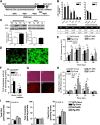
Intramyocellular triacylglycerol (IMTG) accumulation is highly associated with insulin resistance and metabolic complications of obesity (lipotoxicity), whereas comparable IMTG accumulation in endurance-trained athletes is associated with insulin sensitivity (the athlete's paradox). Despite these findings, it remains unclear whether changes in IMTG accumulation and metabolism per se influence muscle-specific and systemic metabolic homeostasis and insulin responsiveness. By mediating the rate-limiting step in triacylglycerol hydrolysis, adipose triglyceride lipase (ATGL) has been proposed to influence the storage/production of deleterious as well as essential lipid metabolites. However, the physiological relevance of ATGL-mediated triacylglycerol hydrolysis in skeletal muscle remains unknown. To determine the contribution of IMTG hydrolysis to tissue-specific and systemic metabolic phenotypes in the context of obesity, we generated mice with targeted deletion or transgenic overexpression of ATGL exclusively in skeletal muscle. Despite dramatic changes in IMTG content on both chow and high-fat diets, modulation of ATGL-mediated IMTG hydrolysis did not significantly influence systemic energy, lipid, or glucose homeostasis, nor did it influence insulin responsiveness or mitochondrial function. These data argue against a role for altered IMTG accumulation and lipolysis in muscle insulin resistance and metabolic complications of obesity.
Figures




Comment in
-
Comment on: Sitnick et al. Skeletal muscle triacylglycerol hydrolysis does not influence metabolic complications of obesity. Diabetes 2013;62:3350-3361.Diabetes. 2013 Dec;62(12):e29. doi: 10.2337/db13-1107. Diabetes. 2013. PMID: 24264409 Free PMC article. No abstract available.
Similar articles
-
Intramuscular triglyceride synthesis: importance in muscle lipid partitioning in humans.Am J Physiol Endocrinol Metab. 2018 Feb 1;314(2):E152-E164. doi: 10.1152/ajpendo.00142.2017. Epub 2017 Oct 3. Am J Physiol Endocrinol Metab. 2018. PMID: 28978544 Free PMC article.
-
Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state.Pflugers Arch. 2006 Feb;451(5):606-16. doi: 10.1007/s00424-005-1509-0. Epub 2005 Sep 10. Pflugers Arch. 2006. PMID: 16155759 Review.
-
Adipose triglyceride lipase deficiency causes tissue-specific changes in insulin signaling.J Biol Chem. 2009 Oct 30;284(44):30218-29. doi: 10.1074/jbc.M109.047787. Epub 2009 Aug 31. J Biol Chem. 2009. PMID: 19723629 Free PMC article.
-
Subcellular localisation and composition of intramuscular triacylglycerol influence insulin sensitivity in humans.Diabetologia. 2021 Jan;64(1):168-180. doi: 10.1007/s00125-020-05315-0. Epub 2020 Oct 31. Diabetologia. 2021. PMID: 33128577 Free PMC article.
-
Dynamics of skeletal muscle lipid pools.Trends Endocrinol Metab. 2013 Dec;24(12):607-15. doi: 10.1016/j.tem.2013.08.001. Epub 2013 Aug 26. Trends Endocrinol Metab. 2013. PMID: 23988586 Review.
Cited by
-
Extremely rapid increase in fatty acid transport and intramyocellular lipid accumulation but markedly delayed insulin resistance after high fat feeding in rats.Diabetologia. 2015 Oct;58(10):2381-91. doi: 10.1007/s00125-015-3691-8. Epub 2015 Jul 22. Diabetologia. 2015. PMID: 26197708
-
Chronic intake of high dietary sucrose induces sexually dimorphic metabolic adaptations in mouse liver and adipose tissue.Nat Commun. 2022 Oct 13;13(1):6062. doi: 10.1038/s41467-022-33840-6. Nat Commun. 2022. PMID: 36229459 Free PMC article.
-
Regulation of Hepatic Triacylglycerol Metabolism by CGI-58 Does Not Require ATGL Co-activation.Cell Rep. 2016 Jul 26;16(4):939-949. doi: 10.1016/j.celrep.2016.06.049. Epub 2016 Jul 7. Cell Rep. 2016. PMID: 27396333 Free PMC article.
-
Peroxisomal β-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis.Nat Metab. 2021 Dec;3(12):1648-1661. doi: 10.1038/s42255-021-00489-2. Epub 2021 Dec 13. Nat Metab. 2021. PMID: 34903883 Free PMC article.
-
PLIN5 deletion remodels intracellular lipid composition and causes insulin resistance in muscle.Mol Metab. 2014 Jun 14;3(6):652-63. doi: 10.1016/j.molmet.2014.06.002. eCollection 2014 Sep. Mol Metab. 2014. PMID: 25161888 Free PMC article.
References
-
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 2003;14:281–287 - PubMed
-
- Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 2001;86:5755–5761 - PubMed
-
- Cowart LA. Sphingolipids: players in the pathology of metabolic disease. Trends Endocrinol Metab 2009;20:34–42 - PubMed
-
- Amati F. Revisiting the diacylglycerol-induced insulin resistance hypothesis. Obes Rev 2012;13(Suppl. 2):40–50 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-DK-090166/DK/NIDDK NIH HHS/United States
- C06-RR-018823/RR/NCRR NIH HHS/United States
- R03 DK077697/DK/NIDDK NIH HHS/United States
- R03-DK-077697/DK/NIDDK NIH HHS/United States
- P20-RR-017677/RR/NCRR NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 CA138313/CA/NCI NIH HHS/United States
- C06 RR018823/RR/NCRR NIH HHS/United States
- R01 DK090166/DK/NIDDK NIH HHS/United States
- P20 RR017677/RR/NCRR NIH HHS/United States
- P30-CA-138313/CA/NCI NIH HHS/United States
- T32 DK007052/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

