Mouse muscle as an ectopic permissive site for human pancreatic development
- PMID: 23835344
- PMCID: PMC3781474
- DOI: 10.2337/db13-0554
Mouse muscle as an ectopic permissive site for human pancreatic development
Abstract
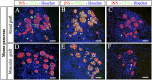
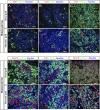
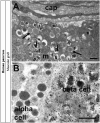
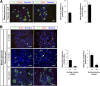
While sporadic human genetic studies have permitted some comparisons between rodent and human pancreatic development, the lack of a robust experimental system has not permitted detailed examination of human pancreatic development. We previously developed a xenograft model of immature human fetal pancreas grafted under the kidney capsule of immune-incompetent mice, which allowed the development of human pancreatic β-cells. Here, we compared the development of human and murine fetal pancreatic grafts either under skeletal muscle epimysium or under the renal capsule. We demonstrated that human pancreatic β-cell development occurs more slowly (weeks) than murine pancreas (days) both by differentiation of pancreatic progenitors and by proliferation of developing β-cells. The superficial location of the skeletal muscle graft and its easier access permitted in vivo lentivirus-mediated gene transfer with a green fluorescent protein-labeled construct under control of the insulin or elastase gene promoter, which targeted β-cells and nonendocrine cells, respectively. This model of engraftment under the skeletal muscle epimysium is a new approach for longitudinal studies, which allows localized manipulation to determine the regulation of human pancreatic development.
Figures



References
-
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 1994;371:606–609 - PubMed
-
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet 1997;15:106–110 - PubMed
-
- Bhushan A, Itoh N, Kato S, et al. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development 2001;128:5109–5117 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

