Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma
- PMID: 23835706
- PMCID: PMC4878050
- DOI: 10.1200/JCO.2012.48.5052
Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma
Abstract
Purpose: Reports detailing the prognostic impact of TP53 mutations in medulloblastoma offer conflicting conclusions. We resolve this issue through the inclusion of molecular subgroup profiles.
Patients and methods: We determined subgroup affiliation, TP53 mutation status, and clinical outcome in a discovery cohort of 397 medulloblastomas. We subsequently validated our results on an independent cohort of 156 medulloblastomas.
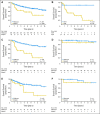
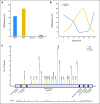
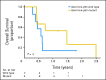
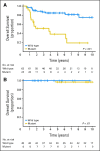
Results: TP53 mutations are enriched in wingless (WNT; 16%) and sonic hedgehog (SHH; 21%) medulloblastomas and are virtually absent in subgroups 3 and 4 tumors (P < .001). Patients with SHH/TP53 mutant tumors are almost exclusively between ages 5 and 18 years, dramatically different from the general SHH distribution (P < .001). Children with SHH/TP53 mutant tumors harbor 56% germline TP53 mutations, which are not observed in children with WNT/TP53 mutant tumors. Five-year overall survival (OS; ± SE) was 41% ± 9% and 81% ± 5% for patients with SHH medulloblastomas with and without TP53 mutations, respectively (P < .001). Furthermore, TP53 mutations accounted for 72% of deaths in children older than 5 years with SHH medulloblastomas. In contrast, 5-year OS rates were 90% ± 9% and 97% ± 3% for patients with WNT tumors with and without TP53 mutations (P = .21). Multivariate analysis revealed that TP53 status was the most important risk factor for SHH medulloblastoma. Survival rates in the validation cohort mimicked the discovery results, revealing that poor survival of TP53 mutations is restricted to patients with SHH medulloblastomas (P = .012) and not WNT tumors.
Conclusion: Subgroup-specific analysis reconciles prior conflicting publications and confirms that TP53 mutations are enriched among SHH medulloblastomas, in which they portend poor outcome and account for a large proportion of treatment failures in these patients.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

References
-
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): Long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. - PubMed
-
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. - PubMed
-
- Grotzer MA, Janss AJ, Fung K, et al. TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol. 2000;18:1027–1035. - PubMed
-
- Ray A, Ho M, Ma J, et al. A clinicobiological model predicting survival in medulloblastoma. Clin Cancer Res. 2004;10:7613–7620. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

