Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer
- PMID: 23835762
- PMCID: PMC6457816
- DOI: 10.1002/14651858.CD006910.pub2
Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer
Update in
-
Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer.Cochrane Database Syst Rev. 2023 Jul 5;7(7):CD006910. doi: 10.1002/14651858.CD006910.pub3. Cochrane Database Syst Rev. 2023. PMID: 37407274 Free PMC article. Review.
Abstract
Background: Ovarian cancer is the eighth most common cancer in women and it is usually diagnosed at an advanced stage. The majority of ovarian tumours are epithelial in origin. Women with relapsed epithelial ovarian cancer (EOC) often have a reduced performance status with a limited life expectancy, therefore maintaining quality of life with effective symptom control is the main purpose of treatment. Drug treatment of relapsed disease is directed by the platinum-free interval: relapsed platinum-sensitive disease is usually re-treated with platinum-based therapy and platinum-resistant disease challenged with non-platinum drugs. However, the side-effects of chemotherapy agents may be severe and optimal treatment regimens are unclear. Pegylated liposomal doxorubicin (PLD), which contains a cytotoxic drug called doxorubicin hydrochloride is one of several treatment modalities that may be considered for single-agent treatment of relapsed EOC, or used in combination with other drugs.
Objectives: To assess the efficacy and safety of PLD in women with relapsed epithelial ovarian cancer (EOC).
Search methods: We searched the Cochrane Gynaecological Cancer Group (CGCG) trials register, CENTRAL, MEDLINE and EMBASE from 1990 to February 2013. We also searched online registers of clinical trials, abstracts of scientific meetings and reference lists of included studies.
Selection criteria: Randomised controlled trials (RCTs) that evaluated PLD in women diagnosed with relapsed epithelial ovarian cancer.
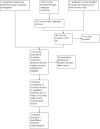
Data collection and analysis: Two review authors independently abstracted data to a pre-designed data collection form and assessed the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions guidelines. Where possible, we pooled collected data in meta-analyses using RevMan 5.2 software.
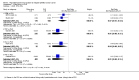
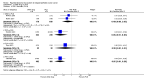
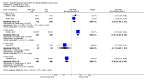
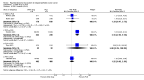
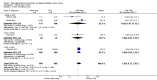
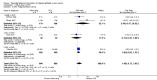
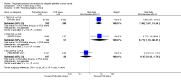
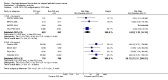
Main results: We included 14 RCTs that evaluated PLD alone or in combination with other drugs. Four RCTs contributed no data to the meta-analyses. Two studies compared PLD plus carboplatin (carbo) to paclitaxel (PAC)/carbo in women with platinum-sensitive relapsed EOC. Overall survival (OS) was similar for these treatments, however progression-free survival (PFS) was longer with PLD/carbo (1164 participants; hazard ratio (HR) 0.85, 95% confidence interval (CI) 0.74 to 0.97; I² = 7%; P value 0.01). PLD/carbo was associated with significantly more anaemia and thrombocytopenia than PAC/carbo, whereas PAC/carbo was associated with significantly more alopecia, neuropathies, hypersensitivity reactions and arthralgias/myalgias. PLD/carbo was well-tolerated and women receiving this treatment were significantly less likely to discontinue treatment than those receiving PAC/carbo (two studies, 1150 participants; risk ratio (RR) 0.38, 95% CI 0.26 to 0.57; I² = 0%; P < 0.00001).Five studies compared other agents to PLD alone. None of these agents were associated with significantly better survival or severe adverse-event profiles than PLD. Topotecan and gemcitabine were associated with significantly more haematological severe adverse events than PLD, and patupilone was associated with significantly more severe neuropathies and diarrhoea. Severe hand-foot syndrome (HFS) occurred consistently more frequently with PLD than the other drugs.Three studies compared PLD combination treatment to PLD alone. Two combinations resulted in a significantly longer PFS compared with PLD alone: trabectedin (TBD)/PLD (one study, 672 women; HR 0.79, 95% CI 0.65 to 0.96; P value 0.02) and vintafolide (EC145)/PLD (one study, 149 women; HR 0.63, 95% CI 0.41 to 0.97; P value 0.04). TBD/PLD appeared to benefit the partially platinum-sensitive subgroup only. Further studies are likely to have an important impact on our confidence in these estimates. TBD/PLD was associated with significantly more haematological and gastrointestinal severe adverse events than PLD alone, whereas EC145/PLD appeared to be well-tolerated.For platinum-resistant relapsed EOC, the median PFS and OS for single-agent PLD across seven included studies was 15 weeks and 54 weeks, respectively. Severe HFS occurred significantly more frequently in women receiving a 50 mg/m² dose of PLD than those receiving less than 50 mg/m² (17% versus 2%, respectively; P value 0.01).
Authors' conclusions: In platinum-sensitive relapsed epithelial ovarian cancer, PLD/carbo is more effective than PAC/carbo and is better tolerated; PLD/carbo should therefore be considered as first-line treatment in women with platinum-sensitive relapsed EOC. PLD alone is a useful agent for platinum-resistant relapsed EOC, however it remains unclear how it compares with other single agents for this subgroup and in what order these agents should be used. There is insufficient evidence to support the use of PLD in combination with other agents in platinum-resistant relapsed EOC.
Conflict of interest statement
None known.
Figures













































References
References to studies included in this review
ASSIST‐3 2007 {published data only}
-
- Rose P, Edwards R, Finkler N, Seiden M, Duska L, Krasner C, et al. Phase 3 Study: Canfosfamide (C, TLK286) plus carboplatin (P) vs liposomal doxorubicin (D) as 2nd line therapy of platinum (P) resistant ovarian cancer (OC). Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007; Vol. 25, 18S (June 20 Supplement), 2007: LBA5529.
ASSIST‐5 2010 {published data only}
-
- Vergote I, Finkler NJ, Hall JB, Melnyk O, Edwards RP, Jones M, et al. Randomized phase III study of canfosfamide in combination with pegylated liposomal doxorubicin compared with pegylated liposomal doxorubicin alone in platinum‐resistant ovarian cancer. International Journal of Gynecological Cancer 2010;20(5):772‐80. - PubMed
-
- Vergote I, et al. Phase 3 study of canfosfamide (TLK 286) plus pegylated liposomal doxorubicin (PLD) vs PLD as second‐line therapy in platinum (P) refractory or resistant ovarian cancer. Journal of Clinical Oncology; 2009 ASCO annual meeting. 2009; Vol. 27(15S): abstr 5552.
CALYPSO 2010 {published and unpublished data}
-
- Alexandre J, Brown C, Priou F, Rauglaudre G, Pfisterer J, Maenpaa J, et al. Should CA 125 still be part of tumour evaluation criteria in ovarian cancer trials? Experience of the GCIG calypso trial. Annals of Oncology, 35th ESMO Congress, Milan, Italy. 2010; Vol. 21(suppl 8):viii 307: abstr. 981P.
-
- Baumann KH, Pujade‐Lauraine E, Jackisch C, Lubbe D, du Bois A, Brown C, et al. Therapy expectations of patients with recurrent platinum‐sensitive ovarian cancer‐a German substudy of the calypso/ago‐ovar 2.9 trial. International Journal of Gynecological Cancer, 17th International Meeting of ESGO. 2011.
-
- Brundage M, Gropp M, Mefti F, Mann K, Lund B, Gebski V, et al. Health‐related quality of life in recurrent platinum‐sensitive ovarian cancer‐‐results from the CALYPSO trial. Annals of Oncology 2012;23(8):2020‐7. - PubMed
-
- Gladieff L, Ferrero A, Rauglaudre G, Brown C, Vasey P, Reinthaller A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum‐sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Annals of Oncology 2012;23(5):1185‐9. - PubMed
Colombo 2012 {published data only}
-
- Colombo N, Kutarska E, Dimopoulos M, Bae DS, Rzepka‐Gorska I, Bidzinski M, et al. Randomized, open‐Label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum‐refractory or ‐resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. Journal of Clinical Oncology 2012;30(31):3841‐7. - PubMed
Gordon 2001 {published data only}
-
- Capri S, Cattaneo G. Cost‐minimization analysis of pegylated liposomal doxorubicin versus topotecan for the treatment of ovarian cancer in Italy. Clinical therapeutics 2003;25(6):1826‐45. - PubMed
-
- Coleman RL, Gordon A, Barter J, Sun S, Rackoff W, Herzog TJ. Early changes in CA125 after treatment with pegylated liposomal doxorubicin or topotecan do not always reflect best response in recurrent ovarian cancer patients. The Oncologist 2007;12(1):72‐8. - PubMed
-
- Gordon A. Overall survival advantage for pegylated liposomal doxorubicin compared to topotecan in recurrent epithelial ovarian cancer [abstract]. European Journal of Cancer; ECCO Conference. 2003; Vol. 1(5):S51.
-
- Gordon A, Sun S, Rackoff W. Incidence of adverse events in women (</=65 or >65 years) with recurrent ovarian cancer receiving pegylated liposomal doxorubicin or topotecan [abstract]. Gynecologic Oncology. 2006; Vol. 101 (1 suppl 1):S59:60.
-
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. Journal of Clinical Oncology 2001;19(14):3312‐22. - PubMed
HeCOG 2010 {published and unpublished data}
-
- Bafaloukos D, Linardou H, Aravantinos G, Papadimitriou G, Bamias A, Fountzilas G, et al. A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study. BMC Medicine 2010;8:3:Accessed on 9/11/2012 at http://www.biomedcentral.com/1741‐7015/8/3. - PMC - PubMed
Kaye 2012 {published data only}
-
- Kaye S, Kaufman B, Lubinski J, Matulonis U, Gourley C, Karlan B, et al. Phase II study of the oral parp inhibitor olaparib (AZD2281) versus liposomal doxorubicin in ovarian cancer patients with BRCA1 and/or BRCA2 mutations. Annals of Oncology; Proceedings of the 35th ESMO Congress. NCT00628251, 2010; Vol. 21(suppl 8, abstr 9710):viii 304.
-
- Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open‐label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP‐ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. Journal of Clinical Oncology 2012;30(4):372‐9. - PubMed
M200 2009 {published data only}
-
- Vergote IB, Colombo N, Kutarska E, Campo J, Pippitt C, Casado A, et al. Phase II study comparing volociximab (an antiangiogenic antibody) and pegylated liposomal doxorubicin (PLD) with PLD alone in recurrent ovarian or primary peritoneal cancer. Journal of Clinical Oncology. 2009; Vol. 27:15s, (suppl; abstr 5560).
MITO‐3 2008 {published and unpublished data}
-
- Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. Journal of Clinical Oncology 2008;26(6):890‐6. - PubMed
Mutch 2007 {published data only}
-
- Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum‐resistant ovarian cancer. Journal of Clinical Oncology 2007;25(19):2811‐8. - PubMed
-
- Mutch DG, Orlando M, Goss T, Wang Y, Lilly E, Teneriello MG, et al. Randomized phase III trial of gemcitabine versus pegylated liposomal doxorubicin for patients with platinum‐resistent taxane‐pretreated ovarian cancer as second or third line therapy [abstract]. Gynecologic Oncology. 2006; Vol. 101:S14 (1 Suppl 1):5.
O'Byrne 2002 {published data only}
-
- O'Byrne KJ, Bliss P, Graham JD, Gerber J, Vasey PA, Khanna S, et al. A phase III study of Doxil/Caelyx versus paclitaxel in platinum‐treated, taxane‐naive relapsed ovarian cancer. Proceedings of the American Society of Clinical Oncologists. 2002; Vol. 21:808.
OVA‐301 2010 {published data only}
-
- Bidzinski M, Poveda A, et al. Influence of an independent review on PFS and response assessments in a phase III clinical trial in relapsed ovarian cancer. European Journal of cancer, Supplement, Proceedings of the Joint 15th ECCO ‐ 34th ESMO Multidisciplinary Congress, Berlin. 2009:var. pagings.
-
- Boman K, Colombo N. Tolerability of trabectedin (TR) plus pegylated liposomal doxorubicin (PLD) in platinum sensitive (p‐s) vs. platinum resistant (P‐R) patients (PTS) with relapsed ovarian cancer. Annals of Oncology; 35th ESMO Congress, Milan, Italy. 2010:var. pagings.
-
- Brundage M, Gropp M, Mefti F, Mann K, Lund B, Gebski V, et al. Health‐related quality of life in recurrent platinum‐sensitive ovarian cancer‐‐results from the CALYPSO trial. Annals of Oncology 2012;23(8):2020‐7. - PubMed
-
- Colombo N. Efficacy of trabectedin in platinum‐sensitive‐relapsed ovarian cancer: new data from the randomized OVA‐301 study. International Journal of Gynecological Cancer 2011;21(Suppl 1):S12‐6. - PubMed
-
- Diebolder H, Runnebaum I. Extending platinum‐free interval (PFI) in partially platinum‐sensitive (PPS) patients (pts) with recurrent ovarian cancer (ROC) treated with trabectedin (Yondelis) plus pegylated liposomal doxorubicin (Caelyx [PLD]) combination versus PLD alone: Results from a PPS cohort of the OVA‐301 phase III study. Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynaecology and Obstetrics (DGGG). 2010.
PRECEDENT 2013 {published data only}
-
- Naumann RW, Coleman RL, Burger RA, Herzog TJ, Morris R, Sausville E, et al. PRECEDENT: A randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in subjects with platinum‐resistant ovarian cancer. Journal of Clinical Oncology. 2011; Vol. 29: (suppl; abstr 5045). - PubMed
-
- Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghaman de SA, et al. PRECENDET: A randomised phase II trial comparing EC145 (Vintafolide) and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in patients with platinum‐resistant ovarian cancer. Unpublished manuscript (submitted to the Journal of Clinical Oncology) 2013 (In press). - PubMed
-
- Naumann RW, Symanowski J, Kutarska E, Sharad G, Nashat G, Pasquale S, et al. EC20 imaging predicts response in a randomized trial comparing pegylated liposomal doxorubicin (PLD) +/‐ EC145 in platinum‐resistant ovarian cancer. International Journal of Gynaecological Cancer, Proceedings of the 17th International Meeting of ESGO. 2011; Vol. 21(suppl 3).
-
- Naumann RW, Symanowski JT, Ghamande SA, Gabrail NY, Gilbert MG, Teneriello G, et al. PRECEDENT: A randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in subjects with platinum‐resistant ovarian cancer [abstract]. Journal of Clinical Oncology, 2010 ASCO Annual Meeting Proceedings. 2010; Vol. 28:18S, (suppl; abstr LBA5012b). - PubMed
SWOG S0200 2008 {published and unpublished data}
-
- Alberts DS, Liu PY, Wilczynski S, Clouser M, Lopez A, Lange M, et al. Phase III randomized trial of pegylated liposomal doxorubicin plus carboplatin versus carboplatin in platinum‐sensitive patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum‐based chemotherapy: Southwest Oncology Group Protocol S0200 [Abstract]. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part 1. 2007; Vol. 25 (18S). - PMC - PubMed
-
- Alberts DS, Liu PY, Wilczynski SP, Clouser MC, Lopez AM, Michelin DP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum‐sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum‐based chemotherapy (Southwest Oncology Group Protocol S0200). Gynecologic Oncology 2008;108(1):90‐4. - PMC - PubMed
-
- Markman M, Moon J, Wilczynski S, Lopez AM, Rowland KM, Michelin DP, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: final survival results of a SWOG (S0200) phase 3 randomized trial. Gynecologic Oncology 2010;116(3):323‐325. - PMC - PubMed
References to studies excluded from this review
ASSIST‐1 2009 {published and unpublished data}
-
- Vergote I, Finkler N, Campo J, Lohr A, Hunter J, Matei D, et al. Single agent, canfosfamide (C, TLK286) vs pegylated liposomal doxorubicin or topotecan in 3rd‐line treatment of platinum refractory or resistant ovarian cancer: phase III study results [Abstract]. Journal of Clinical Oncology; 2007 ASCO Annual Meeting. 2007; Vol. 25:18S (Suppl, abstr. LBA5528).
-
- Vergote, I, Finkler N, Campo J, Lohr A, Hunter J, Matei D, et al. Phase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third‐line therapy in patients with platinum‐refractory or ‐resistant ovarian cancer. European Journal of Cancer 2009;45(13):2324‐32. - PubMed
Cherchi 2003 {published data only}
-
- Cherchi PL, Capobianco G, Fattorini F, Canetto AM, Milia L, Cosso M, et al. Second‐line therapy with topotecan and pegylated liposomal doxorubicin vs. topotecan slone in patients with recurrent ovarian cancer. International Journal of Gynecological Cancer. 2003 IGCS Annual Meeting, 2003; Vol. 13(Suppl):53.
GOG0182/ICON 5 {published data only}
-
- Bookman MA. Erratum. Journal of Clinical Oncology 2009;27(13):2305.
-
- Bookman MA. GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. [Abstract]. Journal of Clinical Oncology. 2006; Vol. 24 (Suppl 18): A‐5002, 256s.
-
- Bookman MA, Greer BE, Ozols RF. Optimal therapy of advanced ovarian cancer: carboplatin and paclitaxel vs. cisplatin and paclitaxel (GOG 158) and an update on GOG0 182‐ICON5. International Journal of Gynecological Cancer 2003;13(6):735‐40. - PubMed
-
- Bookman MA, Gynecologic Cancer InterGroup (GCIG). GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. 2006; Vol. 24, 18S (June 20 Supplement):5002.
Kavanagh 2004 {published data only}
-
- Kavanagh JJ, Garcia A, Choi H, Gershenson DM, Lewis L, Mascavage J, et al. Efficacy of TLK286 (Telcyta) alone and Inparaplatin® or Doxil® (Caelyx®) in platinum resistant ovarian cancer ‐ results from 3 phase 2 studies [abstract]. International Journal of Gynaecological Cancer. 2004; Vol. 14 (suppl 1):abstr 95:28.
MITO‐2 2011 {published data only}
-
- Pignata S, Scambia G, Ferrandina G, Savarese A, Sorio R, Breda E, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first‐line treatment for patients with ovarian cancer: the MITO‐2 randomized phase III trial. Journal of Clinical Oncology 2011;29(27):3628‐35. - PubMed
-
- Pignata S, Scambia G, Savarese A, Breda E, Scollo P, Vivo R, et al. Safety of a 3‐weekly schedule of carboplatin plus pegylatedliposomal doxorubicin as first line chemotherapy in patients withovarian cancer: preliminary results of the MITO‐2 randomized trial. BMC Cancer 2006;6:202:This article is available from: http://www.biomedcentral.com/1471‐2407/6/202. - PMC - PubMed
-
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Pisano C, et al. Carboplatin and pegylated liposomal doxorubicin for advanced ovarian cancer: preliminary activity results of the MITO‐2 phase III trial. Oncology 2009;76(1):49‐54. - PubMed
-
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Vernaglia Lombardi A, et al. Carboplatin plus paclitaxel versus carboplatin plus Stealth liposomal doxorubicin in patients with advanced ovarian cancer: preliminary activity results of the MITO‐2 randomized multicenter trial [Abstract]. Journal of Clinical Oncology; 2007 ASCO Annual meeting. 2007; Vol. 25:18S (abstr. 5532).
-
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Ferrandina G, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): activity and safety results of the MITO‐2 randomized multicenter trial [abstract]. Journal of Clinical Oncology; 2009 ASCO Annual Meeting. 2009; Vol. 27:15S (suppl; abstr LBA5508). - PubMed
Palaia 2006 {published data only}
-
- Palaia I, Angioli R, Calcagno M, Zullo MA, Plotti F, Basile S, et al. Liposome‐encapsulated doxorubicin citrate in previously treated recurrent/metastatic gynecologic malignancies. International Journal of Gynecological Cancer. 2006; Vol. 16(S3): 799. - PubMed
Scarfone 2006 {published data only}
-
- Scarfone, G, Presti M, Scarabelli C, Polverino GP, Polonio N, Bertoglio S, et al. Pegylated liposomal doxorubicin alone or in combination with platinum compounds in recurrent ovarian cancer after first line chemotherapy containing paclitaxel and carboplatin. International Journal of Gynecological Cancer. 2006; Vol. 16(S3):668.
References to ongoing studies
ABT‐888/NCT01113957 {published data only}
-
- Abbott. A trial of ABT‐888 in combination with temozolomide versus pegylated liposomal doxorubicin alone in ovarian cancer [Ongoing]. Accessed on 12/11/12 at http://www.http://clinicaltrials.gov/show/NCT01113957.
AGOG06‐001 {published data only}
-
- Lai C‐H. Phase III randomised trial of maintenance pegylated liposomal doxorubicin/carboplatin versus without in patients with advanced ovarian cancer [Ongoing]. Australia and New Zealand Clinical Trials Registry; 2010; Vol. ACTRN12607000329460:Accessed on 20/11/12 at http://www.anzctr.org.au/ACTRN12607000329460.aspx.
ATI0918/NCT01715168 {published data only}
-
- Kuhn K. A Crossover bioequivalence study of intravenously administered ATI0918 and DOXIL/CAELYX in patients with ovarian cancer. Accessed on 14/11/12 at ttp://www.clinicaltrials.gov/ct2/show/record/NCT01715168.
AURELIA {published data only}
-
- Hoffmann‐La Roche. AURELIA: A study of Avastin (Bevacizumab) added to chemotherapy in patients with platinum‐resistant ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT00976911.
HECTOR {published data only}
-
- Meier W, Lichtenegger W, Marth C, Gonzalez‐Martin AJ, Harter P, Tome O, et al. Topotecan plus carboplatin versus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabin plus carboplatin (GC) or carboplatin plus pegylated doxorubicin (PLDC): A planed 200‐pt interim safety analysis of the NOGGO‐AGO‐Germany‐AGO Austria and GEICO‐GCIG Intergroup Study (HECTOR). Journal of Clinical Oncology, 2010 ASCO Annual Meeting. 2010; Vol. 28:15s, (suppl; abstr 5071).
-
- Sehouli J, Meier W, Wimberger P, Chekerov R, Belau A, Mahner S, et al. Topotecan plus carboplatin vesus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabin plus carboplatin (GC) or carboplatin plus pegylated doxorubicin (PLDC): a randomized phase III trial of the NOGGO‐AGO‐Germany‐AGO Austria and GEICO‐GCIG intergroup study (HECTOR) [abstract]. Journal of Clinical Oncology, 2012 ASCO Annual Meeting. 2012; Vol. 30 (suppl, abstr 5031). - PubMed
IMC‐383/NCT00913835 {published data only}
-
- ImClone. A study of liposomal doxorubicin with or without IMC‐3G3 in platinum‐refractory or resistant advanced ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT00913835.
-
- McGuire WP, Shah GD, Loizos N, Youssoufian H, Rowinsky EK, Gore ME, et al. Randomized phase II trial of pegylated liposomal doxorubicin (PLD) with or without anti‐platelet‐derived growth factor receptor‐alpha (PDGFR‐alpha) monoclonal antibody IMC‐3G3 in platinum‐refractory/resistant advanced ovarian cancer. Journal of Clinical Oncology. 2010; Vol. 28:15s, (suppl; abstr TPS256).
INOVATYON {published data only}
-
- Colombo N. INOVATYON STUDY ‐International, randomized study in patients with ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01379989.
MITO‐8 {published data only}
-
- NCT00657878. Liposomal doxorubicin versus carboplatin/paclitaxel in patients with ovarian cancer recurrence between 6 and 12 months after previous platinum based therapy: Phase III randomized multicenter study [Ongoing]. Accessed 13/11/12 at http://clinicaltrials.gov/ct2/show/study/NCT00657878.
NCT01100372 {published data only}
-
- Zeimet A. Randomized phase II AGO‐study comparing combined liposomal doxorubicin (Myocet) and gemcitabine (Gemzar) with liposomal doxorubicin (Myocet) monotherapy in platinum‐refractory and platinum‐resistant epithelial cancer of the ovary, fallopian tube and the peritoneum. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01100372.
NCT01666444 {published data only}
-
- Monk B. VTX‐2337 and pegylated liposomal doxorubicin (PLD) in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer. Accessed on 14/11/12 at http://clinicaltrials.gov/ct2/show/record/NCT01666444.
NGR018 {published data only}
-
- MolMed. Phase II study of NGR‐hTNF in combination with doxorubicin in platinum‐resistant ovarian cancer (NGR018). Accessed on 14/11/12 at http://clinicaltrials.gov/show/NCT01358071.
PROCEED {published data only}
-
- Endocyte. Study for women with platinum resistant ovarian cancer evaluating EC145 in combination with doxil. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01170650.
PROVE {published data only}
-
- Sehouli J. PROVE A randomized phase II trial of standard carboplatin‐based chemotherapy with or without panitumumab in platinum‐sensitive recurrent ovarian cancer. Accessed on 14/11/12 at http://clinicaltrials.gov/ct2/show/NCT01388621.
SGI‐110/NCT01696032 {published data only}
-
- Medpace Recruitment Center. A randomized, controlled, open‐label, phase 2 trial of SGI‐110 and carboplatin in subjects with platinum‐resistant recurrent ovarian cancer. accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01696032.
TRINOVA‐2 {published data only}
-
- AMGEN. Trial IN OVArian cancer‐2. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01281254.
Volasertib/NCT01121406 {published data only}
-
- Boehringer Ingelheim Pharmaceuticals. Phase II randomized trial of the polo‐like kinase 1 inhibitor BI 6727 (Volasertib) monotherapy versus investigator´s choice chemotherapy in ovarian cancer patients resistant or refractory to platinum‐based cytotoxic therapy [Ongoing]. Accessed on 12/11/12 at http://clinicaltrials.gov/ct2/show/NCT01121406.
Additional references
CAELYX PI
-
- Janssen‐Cilag Pty, Ltd. CAELYX Product Information. http://www.janssen.com.au/Products/Caelyx Amended 4 January 2011:(Accessed 24 January 2013).
CTCAE 2006
-
- CTCAE. Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining for heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith g, Altman DG editor(s). Systematic Reviews in Healthcare: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001.
Dersimonian 1986
-
- Dersimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
DOXIL 2011
-
- Janssen Products. Important update regarding the availability of DOXIL. http://www.doxil.com/sites/default/files/DOXIL_DHCP_letter_July2011.pdf 2011:(Accessed 24/1/13).
EMA 2010
-
- European Medicines Agency. EPARs for authorised medicinal products for human use EPARs for authorised medicinal products for human use. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for... 2010; Vol. accessed 24 January 2013.
ESMO 2010
-
- Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, et al. on behalf of the ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2010;21(suppl 5):v23‐30. - PubMed
EUROCARE 2003
-
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14 (Supplement 5):v61‐v118. - PubMed
Farr 2012
Ferlay 2013
-
- Ferlay J. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. European Journal of Cancer 2013;49(6):1374‐403. - PubMed
FIGO 2009
-
- FIGO Committee in Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics 2009;105:3‐4. - PubMed
Gabizon 2001
-
- Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clinical Cancer Research 2001;7(2):223‐5. - PubMed
Gladieff 2012
-
- Gladieff L, Ferrero A, Rauglaudre G, Brown C, Vasey P, Reinthaller A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum‐sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Annals of Oncology 2012;23(5):1185‐9. - PubMed
GLOBOCAN 2008
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10 [Internet]. Lyon, France, 2010; Vol. International Agency for Research on Cancer:http://globocan.iarc.fr.
Hennessy 2009
-
- Hennessy BT, Coleman RL, Markman M. Ovarian Cancer. Lancet 2009;374:1371‐82. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICBP 2012
-
- Maringe C, Walters S, Butler J, Coleman MP, Hacker N, Hanna L, et al. Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecologic Oncology 2012;127(1):75‐82. - PubMed
ICON‐4
-
- Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum‐based chemotherapy versus conventional platinum‐based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO‐OVAR‐2.2 trial. Lancet 2003;361:2099–106. - PubMed
Jemal 2008
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA: A Cancer Journal for Clinicians 2008;58:71‐96. - PubMed
Jemel 2011
-
- Jemel A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer Journal 2011;61(2):69‐90. - PubMed
Kavanagh 2010
-
- Kavanagh JJ, Levenbach CF, Ramires PT, Wolf JL, Moore CL, Jones MR, et al. Phase 2 study of canfosfamide in combination with pegylated liposomal doxorubicin in platinum and paclitaxel refractory or resistant epithelial ovarian cancer. Journal of Hematology & Oncology; www.jhoonline.org/content/3/1/9 2010;3:9. - PMC - PubMed
Lorusso 2007
-
- Lorusso D, Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin‐related palmar‐plantar erthrodysesthesia ('hand‐foot syndrome'). Annals of Oncology 2007;18(7):1159‐64. - PubMed
Mangili 2008
-
- Mangili G, Petrone M, Gentile C, Marzi P, Vigano R, Rabaiotti E. Prevention strategies in palmar‐plantar erythodysesthesia onset: the role of regional cooling. Gynecologic Oncology 2008;108:332‐5. - PubMed
Markman 2010
-
- Markman M. Serious ethical dilemma of single‐agent peylyated liposomal doxorubin employed as a control arm in ovarian cancer chemotherapy trials. Journal of Clinical Oncology 2010;28(19):e319‐20. - PubMed
Monk 2010
-
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. Journal of Clinical Oncology 2010;28(19):3107‐14. - PubMed
Monk 2012
-
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. European Journal of Cancer 2012;48(15):2361‐8. - PubMed
Naumann 2011
-
- Naumann RW, Coleman RL. Management strategies for recurrent platinum‐resistant ovarian cancer. Drugs 2011;71:1397‐412. - PubMed
NICE 2003
-
- National Institute for Clinical Excellence. Guidance on the use of paclitaxel in the treatment of ovarian cancer. NICE: Technology Appraisal Guidance‐ No. 55 2003.
NICE 2005
-
- National Institute for Health and Clinical Excellence. Paclitaxel, pegylated liposomal doxorubicin hydrochloride and topotecan for second‐line or subsequent treatment of advanced ovarian cancer (review). www.nice.org.uk/nicemedia/live/11554/33026/33026.pdf 2005:1‐4. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Pfisterer 2006
-
- Pfisterer J, Lederman JA. Management of platinum‐sensitive recurrent ovarian cancer. Seminars in Oncology 2006;33(2 Suppl 6):S12‐6. - PubMed
Poveda 2011
-
- Poveda A, Vergote I, Tjulandin S, Kong B, Roy M, Chan S, et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum‐sensitive (platinum‐free interval 6‐12 months) subpopulation of OVA‐301 phase III randomized trial. Annals of Oncology 2011;22(1):39‐48. - PMC - PubMed
RevMan 5.2 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rose 2005
-
- Rose PG. Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarian cancer. Oncologist 2005;10:205‐14. - PubMed
Rustin 1996
-
- Rustin GJ, Nelstrop AE, Tuxen MK, Lambert HE. Defining progression of ovarian carcinoma during follow‐up according to CA 125: a North Thames Ovary Group Study. Annals of Oncology 1996;7:361‐4. - PubMed
SEER 2007
-
- Kosary CL. Chapter 16: Cancer of the ovary. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M‐J editor(s). Cancer Survival Among Adults: US SEER Program, 1988‐2001. Bethesda: US Department of Health and Human Services; National Cancer Institute, 2007:133‐144.
Theodoulou 2004
-
- Theodoulou M, Hudis C. Cardiac profiles of liposomal anthracyclines. Cancer 2004;100(10):2052‐63. - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumours: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92:205‐16. - PubMed
von Moos 2008
-
- Moos R, Thuerlimann BJK, Aapro M, Rayson D, Harrold K, Sehouli J, et al. Pegylated liposomal doxorubicin‐associated hand‐foot syndrome: recommendations of an international panel of experts. European Journal of Cancer 2008;44:781‐90. - PubMed
Wagner 2012
-
- Wagner U, Marth C, Largillier R, Kaern J, Brown C, Heywood M, et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum‐sensitive ovarian cancer patients. British Journal of Cancer 2012;107(4):588‐91. - PMC - PubMed
Zunino 2002
-
- Zunino F, Pratesi G. Antitumour Antibodies. In: Souhami RL, Tannock I, Hohenberger P, Horiot J‐C editor(s). Oxford Textbook of Oncology. 2nd Edition. Oxford: Oxford University Press, 2002:715‐27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

