Corticotropin-releasing factor receptor 2 mediates sex-specific cellular stress responses
- PMID: 23835907
- PMCID: PMC3745594
- DOI: 10.2119/molmed.2013.00036
Corticotropin-releasing factor receptor 2 mediates sex-specific cellular stress responses
Abstract
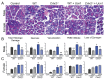
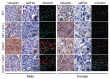
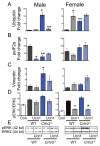
Although females suffer twice as much as males from stress-related disorders, sex-specific participating and pathogenic cellular stress mechanisms remain uncharacterized. Using corticotropin-releasing factor receptor 2-deficient (Crhr2-/-) and wild-type (WT) mice, we show that CRF receptor type 2 (CRF2) and its high-affinity ligand, urocortin 1 (Ucn1), are key mediators of the endoplasmic reticulum (ER) stress response in a murine model of acute pancreatic inflammation. Ucn1 was expressed de novo in acinar cells of male, but not female WT mice during acute inflammation. Upon insult, acinar Ucn1 induction was markedly attenuated in male but not female Crhr2-/- mice. Crhr2-/- mice of both sexes show exacerbated acinar cell inflammation and necrosis. Electron microscopy showed mild ER damage in WT male mice and markedly distorted ER structure in Crhr2-/- male mice during pancreatitis. WT and Crhr2-/- female mice showed similarly distorted ER ultrastructure that was less severe than distortion seen in Crhr2-/- male mice. Damage in ER structure was accompanied by increased ubiquitination, peIF2, and mistargeted localization of vimentin in WT mice that was further exacerbated in Crhr2-/- mice of both sexes during pancreatitis. Exogenous Ucn1 rescued many aspects of histological damage and cellular stress response, including restoration of ER structure in male WT and Crhr2-/- mice, but not in females. Instead, females often showed increased damage. Thus, specific cellular pathways involved in coping and resolution seem to be distinct to each sex. Our results demonstrate the importance of identifying sex-specific pathogenic mechanisms and their value in designing effective therapeutics.
Figures




References
-
- Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–32. - PubMed
-
- Slominski A, et al. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–93. - PubMed
-
- Chen A, Blount A, Vaughan J, Brar B, Vale W. Urocortin II gene is highly expressed in mouse skin and skeletal muscle tissues: localization, basal expression in corticotropin-releasing factor receptor (CRFR) 1- and CRFR2-null mice, and regulation by glucocorticoids. Endocrinology. 2004;145:2445–57. - PubMed
-
- Baigent SM. Peripheral corticotropin-releasing hormone and urocortin in the control of the immune response. Peptides. 2001;22:809–20. - PubMed
-
- Wu Y, Xu Y, Zhou H, Tao J, Li S. Expression of urocortin in rat lung and its effect on pulmonary vascular permeability. J Endocrinol. 2006;189:167–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

