Current progress in development of hepatitis C virus vaccines
- PMID: 23836237
- PMCID: PMC6263146
- DOI: 10.1038/nm.3183
Current progress in development of hepatitis C virus vaccines
Abstract
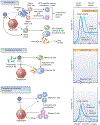
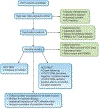
Despite major advances in the understanding and treatment of hepatitis C, a preventive vaccine remains elusive. The marked genetic diversity and multiple mechanisms of persistence of hepatitis C virus, combined with the relatively poor immune response of the infected host against the virus, are major barriers. The lack of robust and convenient model systems further hampers the effort to develop an effective vaccine. Advances in our understanding of virus-host interactions and protective immunity in hepatitis C virus infection provide an important roadmap to develop potent and broadly directed vaccine candidates targeting both humoral and cellular immune responses. Multiple approaches to generating and testing viral immunogens have met with variable success. Several candidates have advanced to clinical trials based on promising results in chimpanzees. The ultimate path to a successful preventive vaccine requires comprehensive evaluations of all aspects of protective immunity, innovative application of state-of-the-art vaccine technology and properly designed vaccine trials that can affirm definitive endpoints of efficacy.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
Figures

References
-
- Choo QL et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244, 359–362 (1989). - PubMed
-
- Liang TJ, Rehermann B, Seeff LB & Hoofnagle JH Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med 132, 296–305 (2000). - PubMed
-
- Bowen DG & Walker CM Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436, 946–952 (2005). - PubMed
-
- Lindenbach BD & Rice CM Unravelling hepatitis C virus replication from genome to function. Nature 436, 933–938 (2005). - PubMed
-
- Hoofnagle JH & Seeff LB Peginterferon and ribavirin for chronic hepatitis C. N. Engl. J. Med 355, 2444–2451 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

