Regulation of hepatic innate immunity by hepatitis C virus
- PMID: 23836238
- PMCID: PMC4251871
- DOI: 10.1038/nm.3253
Regulation of hepatic innate immunity by hepatitis C virus
Abstract
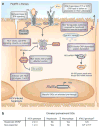
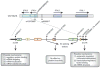
Hepatitis C virus (HCV) is a global public health problem involving chronic infection of the liver, which can cause liver disease and is linked with liver cancer. Viral innate immune evasion strategies and human genetic determinants underlie the transition of acute HCV infection to viral persistence and the support of chronic infection. Host genetic factors, such as sequence polymorphisms in IFNL3, a gene in the host interferon system, can influence both the outcome of the infection and the response to antiviral therapy. Recent insights into how HCV regulates innate immune signaling within the liver reveal a complex interaction of patient genetic background with viral and host factors of innate immune triggering and control that imparts the outcome of HCV infection and immunity.
Figures



References
-
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. - PubMed
-
- Soriano V, Peters MG, Zeuzem S. New therapies for hepatitis C virus infection. Clin Infect Dis. 2009;48:313–320. - PubMed
-
- Hofmann WP, Zeuzem S. A new standard of care for the treatment of chronic HCV infection. Nat Rev Gastroenterol Hepatol. 2011;8:257–264. - PubMed
-
- Sarrazin C, Hezode C, Zeuzem S, Pawlotsky JM. Antiviral strategies in hepatitis C virus infection. J Hepatol. 2012;56(Suppl):S88–S100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

