Activation loop phosphorylation of a protein kinase is a molecular marker of organelle size that dynamically reports flagellar length
- PMID: 23836633
- PMCID: PMC3725086
- DOI: 10.1073/pnas.1302364110
Activation loop phosphorylation of a protein kinase is a molecular marker of organelle size that dynamically reports flagellar length
Abstract
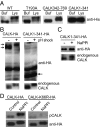
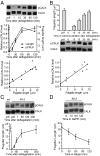
Specification of organelle size is crucial for cell function, yet we know little about the molecular mechanisms that report and regulate organelle growth and steady-state dimensions. The biflagellated green alga Chlamydomonas requires continuous-length feedback to integrate the multiple events that support flagellar assembly and disassembly and at the same time maintain the sensory and motility functions of the organelle. Although several length mutants have been characterized, the requisite molecular reporter of length has not been identified. Previously, we showed that depletion of Chlamydomonas aurora-like protein kinase CALK inhibited flagellar disassembly and that a gel-shift-associated phosphorylation of CALK marked half-length flagella during flagellar assembly. Here, we show that phosphorylation of CALK on T193, a consensus phosphorylation site on the activation loop required for kinase activity, is distinct from the gel-shift-associated phosphorylation and is triggered when flagellar shortening is induced, thereby implicating CALK protein kinase activity in the shortening arm of length control. Moreover, CALK phosphorylation on T193 is dynamically related to flagellar length. It is reduced in cells with short flagella, elevated in the long flagella mutant, lf4, and dynamically tracks length during both flagellar assembly and flagellar disassembly in WT, but not in lf4. Thus, phosphorylation of CALK in its activation loop is implicated in the disassembly arm of a length feedback mechanism and is a continuous and dynamic molecular marker of flagellar length during both assembly and disassembly.
Keywords: aurora kinase; cilia and flagella; cilia length; flagellar length control; organelle size control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wemmer KA, Marshall WF. Flagellar length control in chlamydomonas—paradigm for organelle size regulation. Int Rev Cytol. 2007;260:175–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

