Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy
- PMID: 23836662
- PMCID: PMC3725072
- DOI: 10.1073/pnas.1222684110
Loss of corepressor PER2 under hypoxia up-regulates OCT1-mediated EMT gene expression and enhances tumor malignancy
Abstract
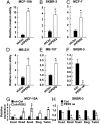
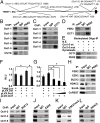
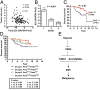
The circadian clock gene Period2 (PER2) has been suggested to be a tumor suppressor. However, detailed mechanistic evidence has not been provided to support this hypothesis. We found that loss of PER2 enhanced invasion and activated expression of epithelial-mesenchymal transition (EMT) genes including TWIST1, SLUG, and SNAIL. This finding was corroborated by clinical observation that PER2 down-regulation was associated with poor prognosis in breast cancer patients. We further demonstrated that PER2 served as a transcriptional corepressor, which recruited polycomb proteins EZH2 and SUZ12 as well as HDAC2 to octamer transcription factor 1 (OCT1) (POU2F1) binding sites of the TWIST1 and SLUG promoters to repress expression of these EMT genes. Hypoxia, a condition commonly observed in tumors, caused PER2 degradation and disrupted the PER2 repressor complex, leading to activation of EMT gene expression. This result was further supported by clinical data showing a significant negative correlation between hypoxia and PER2. Thus, our findings clearly demonstrate the tumor suppression function of PER2 and elucidate a pathway by which hypoxia promotes EMT via degradation of PER2.
Keywords: HIF1alpha; breast cancer stem cell.
Conflict of interest statement
Conflict of interest statement: According to the UCI policy, W.-H.L. declares that he serves as a member of Board of Directors of the biotech company, GeneTex. This arrangement has been reviewed and approved by UCI conflict of interest committee.
Figures



References
-
- Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: Implications for biological rhythms. J Biol Rhythms. 2001;16(3):196–204. - PubMed
-
- Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8(10):790–802. - PubMed
-
- Kuhlman SJ, McMahon DG. Encoding the ins and outs of circadian pacemaking. J Biol Rhythms. 2006;21(6):470–481. - PubMed
-
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

