Cardiac calcium signalling pathologies associated with defective calmodulin regulation of type 2 ryanodine receptor
- PMID: 23836685
- PMCID: PMC3779117
- DOI: 10.1113/jphysiol.2013.256123
Cardiac calcium signalling pathologies associated with defective calmodulin regulation of type 2 ryanodine receptor
Abstract
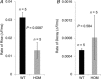
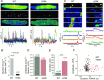
Cardiac ryanodine receptor (RyR2) is a homotetramer of 560 kDa polypeptides regulated by calmodulin (CaM), which decreases its open probability at diastolic and systolic Ca(2+) concentrations. Point mutations in the CaM-binding domain of RyR2 (W3587A/L3591D/F3603A, RyR2(ADA)) in mice result in severe cardiac hypertrophy, poor left ventricle contraction and death by postnatal day 16, suggesting that CaM inhibition of RyR2 is required for normal cardiac function. Here, we report on Ca(2+) signalling properties of enzymatically isolated, Fluo-4 dialysed whole cell clamped cardiac myocytes from 10-15-day-old wild-type (WT) and homozygous Ryr2(ADA/ADA) mice. Spontaneously occurring Ca(2+) spark frequency, measured at -80 mV, was 14-fold lower in mutant compared to WT myocytes. ICa, though significantly smaller in mutant myocytes, triggered Ca(2+) transients that were of comparable size to those of WT myocytes, but with slower activation and decay kinetics. Caffeine-triggered Ca(2+) transients were about three times larger in mutant myocytes, generating three- to four-fold bigger Na(+)-Ca(2+) exchanger NCX currents (INCX). Mutant myocytes often exhibited Ca(2+) transients of variable size and duration that were accompanied by similarly alternating and slowly activating INCX. The data suggest that RyR2(ADA) mutation produces significant reduction in ICa density and ICa-triggered Ca(2+) release gain, longer but infrequently occurring Ca(2+) sparks, larger sarcoplasmic reticulum Ca(2+) loads, and spontaneous Ca(2+) releases accompanied by activation of large and potentially arrhythmogenic inward INCX.
Figures






References
-
- Anderson ME, Braun AP, Schulman H, Premack BA. Multifunctional Ca2+/calmodulin-dependent protein kinase mediates Ca(2+)-induced enhancement of the L-type Ca2+ current in rabbit ventricular myocytes. Circ Res. 1994;75:854–861. - PubMed
-
- Balshaw DM, Yamaguchi N, Meissner G. Modulation of intracellular calcium-release channels by calmodulin. J Membr Biol. 2002;185:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

