Length dependence of striated muscle force generation is controlled by phosphorylation of cTnI at serines 23/24
- PMID: 23836688
- PMCID: PMC3784197
- DOI: 10.1113/jphysiol.2013.258400
Length dependence of striated muscle force generation is controlled by phosphorylation of cTnI at serines 23/24
Abstract
According to the Frank-Starling relationship, greater end-diastolic volume increases ventricular output. The Frank-Starling relationship is based, in part, on the length-tension relationship in cardiac myocytes. Recently, we identified a dichotomy in the steepness of length-tension relationships in mammalian cardiac myocytes that was dependent upon protein kinase A (PKA)-induced myofibrillar phosphorylation. Because PKA has multiple myofibrillar substrates including titin, myosin-binding protein-C and cardiac troponin I (cTnI), we sought to define if phosphorylation of one of these molecules could control length-tension relationships. We focused on cTnI as troponin can be exchanged in permeabilized striated muscle cell preparations, and tested the hypothesis that phosphorylation of cTnI modulates length dependence of force generation. For these experiments, we exchanged unphosphorylated recombinant cTn into either a rat cardiac myocyte preparation or a skinned slow-twitch skeletal muscle fibre. In all cases unphosphorylated cTn yielded a shallow length-tension relationship, which was shifted to a steep relationship after PKA treatment. Furthermore, exchange with cTn having cTnI serines 23/24 mutated to aspartic acids to mimic phosphorylation always shifted a shallow length-tension relationship to a steep relationship. Overall, these results indicate that phosphorylation of cTnI serines 23/24 is a key regulator of length dependence of force generation in striated muscle.
Figures


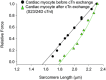

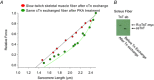



Comment in
-
Cardiac troponin I phosphorylation and the force-length relationship.J Physiol. 2013 Dec 15;591(24):6135-6. doi: 10.1113/jphysiol.2013.265090. J Physiol. 2013. PMID: 24339151 Free PMC article. No abstract available.
References
-
- Biesiadecki BJ, Kobayashi T, Walker JS, Solaro RJ, de Tombe PP. The troponin C G159D mutation blunts myofilament desensitization induced by troponin I Ser23/24 phosphorylation. Circ Res. 2007;100:1486–1493. - PubMed
-
- Chandra M, Kim JJ, Solaro RJ. An improved method for exchanging troponin subunits in detergent skinned rat cardiac fibre bundles. Biochem Biophys Res Commun. 1999;263:219–223. - PubMed
-
- De Tombe PP, Stienen GJM. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ Res. 1995;76:734–741. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

