An expanded tool kit for the auxin-inducible degron system in budding yeast
- PMID: 23836714
- PMCID: PMC4171812
- DOI: 10.1002/yea.2967
An expanded tool kit for the auxin-inducible degron system in budding yeast
Abstract
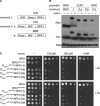
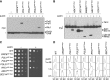
Fusion of inducible degradation signals, so-called degrons, to cellular proteins is an elegant method of controlling protein levels in vivo. Recently, a degron system relying on the plant hormone auxin has been described for use in yeast and vertebrate cells. We now report the construction of a series of vectors that significantly enhance the versatility of this auxin-inducible degron (AID) system in Saccharomyces cerevisiae. We have minimized the size of the degron and appended a series of additional epitope tags, allowing detection by commercial antibodies or fluorescence microscopy. The vectors are compatible with PCR-based genomic tagging strategies, allow for C- or N-terminal fusion of the degron, and provide a range of selection markers. Application to a series of yeast proteins, including essential replication factors, provides evidence for a general usefulness of the system.
Keywords: auxin; budding yeast; degron; protein degradation; protein stability.
Copyright © 2013 John Wiley & Sons, Ltd.
Figures



Similar articles
-
Auxin-Inducible Degron System for Depletion of Proteins in Saccharomyces cerevisiae.Curr Protoc Mol Biol. 2019 Sep;128(1):e104. doi: 10.1002/cpmb.104. Curr Protoc Mol Biol. 2019. PMID: 31503416 Free PMC article.
-
iAID: an improved auxin-inducible degron system for the construction of a 'tight' conditional mutant in the budding yeast Saccharomyces cerevisiae.Yeast. 2015 Aug;32(8):567-81. doi: 10.1002/yea.3080. Epub 2015 Jul 14. Yeast. 2015. PMID: 26081484
-
Quantitative characterization of the auxin-inducible degron: a guide for dynamic protein depletion in single yeast cells.Sci Rep. 2017 Jul 5;7(1):4704. doi: 10.1038/s41598-017-04791-6. Sci Rep. 2017. PMID: 28680098 Free PMC article.
-
Auxin-inducible degron system: an efficient protein degradation tool to study protein function.Biotechniques. 2023 Apr;74(4):186-198. doi: 10.2144/btn-2022-0108. Epub 2023 May 16. Biotechniques. 2023. PMID: 37191015 Review.
-
Rapid Depletion of Budding Yeast Proteins via the Fusion of an Auxin-Inducible Degron (AID).Curr Protoc Cell Biol. 2014 Sep 2;64:20.9.1-16. doi: 10.1002/0471143030.cb2009s64. Curr Protoc Cell Biol. 2014. PMID: 25181302 Review.
Cited by
-
A dual molecular analogue tuner for dissecting protein function in mammalian cells.Nat Commun. 2016 May 27;7:11742. doi: 10.1038/ncomms11742. Nat Commun. 2016. PMID: 27230261 Free PMC article.
-
1,10-phenanthroline inhibits sumoylation and reveals that yeast SUMO modifications are highly transient.EMBO Rep. 2024 Jan;25(1):68-81. doi: 10.1038/s44319-023-00010-8. Epub 2024 Jan 5. EMBO Rep. 2024. PMID: 38182817 Free PMC article.
-
Phosphorylation by the stress-activated MAPK Slt2 down-regulates the yeast TOR complex 2.Genes Dev. 2018 Dec 1;32(23-24):1576-1590. doi: 10.1101/gad.318709.118. Epub 2018 Nov 26. Genes Dev. 2018. PMID: 30478248 Free PMC article.
-
Linkage reprogramming by tailor-made E3s reveals polyubiquitin chain requirements in DNA-damage bypass.Mol Cell. 2022 Apr 21;82(8):1589-1602.e5. doi: 10.1016/j.molcel.2022.02.016. Epub 2022 Mar 8. Mol Cell. 2022. PMID: 35263628 Free PMC article.
-
Plasma Membrane Association by N-Acylation Governs PKG Function in Toxoplasma gondii.mBio. 2017 May 2;8(3):e00375-17. doi: 10.1128/mBio.00375-17. mBio. 2017. PMID: 28465425 Free PMC article.
References
-
- Branzei D, Foiani M. The Rad53 signal transduction pathway: replication fork stabilization, DNA repair, and adaptation. Exp Cell Res. 2006;312:2654–2659. - PubMed
-
- Carthew RW. Gene silencing by double-stranded RNA. Curr Opin Cell Biol. 2001;13:244–248. - PubMed
-
- Dohmen RJ, Wu P, Varshavsky A. Heat-inducible degron: a method for constructing temperature-sensitive mutants. Science. 1994;263:1273–1276. - PubMed
-
- Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell. 1987;48:1035–1046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

