Active plasma membrane P-type H+-ATPase reconstituted into nanodiscs is a monomer
- PMID: 23836891
- PMCID: PMC3772188
- DOI: 10.1074/jbc.M112.446948
Active plasma membrane P-type H+-ATPase reconstituted into nanodiscs is a monomer
Abstract
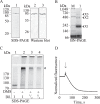
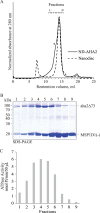
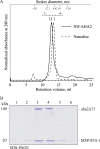
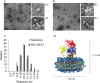
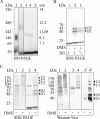
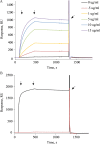
Plasma membrane H(+)-ATPases form a subfamily of P-type ATPases responsible for pumping protons out of cells and are essential for establishing and maintaining the crucial transmembrane proton gradient in plants and fungi. Here, we report the reconstitution of the Arabidopsis thaliana plasma membrane H(+)-ATPase isoform 2 into soluble nanoscale lipid bilayers, also termed nanodiscs. Based on native gel analysis and cross-linking studies, the pump inserts into nanodiscs as a functional monomer. Insertion of the H(+)-ATPase into nanodiscs has the potential to enable structural and functional characterization using techniques normally applicable only for soluble proteins.
Keywords: H+-ATPase; Monomer; Nanodiscs; Plasma Membrane Proton Pumps; Reconstitution of Membrane Transporters; Scaffold Proteins; Surface Plasmon Resonance (SPR).
Figures


References
-
- Axelsen K. B., Palmgren M. G. (1998) Evolution of substrate specificities in the P-type ATPase superfamily. J. Mol. Evol. 46, 84–101 - PubMed
-
- Palmgren M. G., Nissen P. (2011) P-type ATPases. Annu. Rev. Biophys. 40, 243–266 - PubMed
-
- Hager A. (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J. Plant Res. 116, 483–505 - PubMed
-
- Duby G., Poreba W., Piotrowiak D., Bobik K., Derua R., Waelkens E., Boutry M. (2009) Activation of plant plasma membrane H+-ATPase by 14-3-3 proteins is negatively controlled by two phosphorylation sites within the H+-ATPase C-terminal region. J. Biol. Chem. 284, 4213–4221 - PubMed
-
- Fuglsang A. T., Paez-Valencia J., Gaxiola R. A. (2011) in Plant Proton Pumps: Regulatory Circuits Involving H+-ATPase and H+-PPase (Geisler M., Venema K., eds) DOI 10.1007/978-3-642-14369-4_2, Springer-Verlag, Berlin/Heidelberg, Germany - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

