Maternal embryonic leucine zipper kinase (MELK) reduces replication stress in glioblastoma cells
- PMID: 23836907
- PMCID: PMC3745365
- DOI: 10.1074/jbc.M113.471433
Maternal embryonic leucine zipper kinase (MELK) reduces replication stress in glioblastoma cells
Retraction in
-
Maternal embryonic leucine zipper kinase (MELK) reduces replication stress in glioblastoma cells.J Biol Chem. 2017 Aug 4;292(31):12786. doi: 10.1074/jbc.A113.471433. J Biol Chem. 2017. PMID: 28778883 Free PMC article. No abstract available.
Abstract
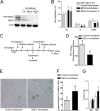
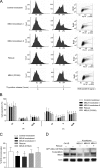
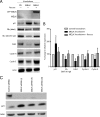
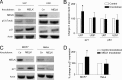
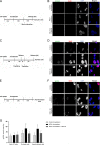
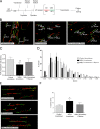
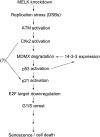
Maternal embryonic leucine zipper kinase (MELK) belongs to the subfamily of AMP-activated Ser/Thr protein kinases. The expression of MELK is very high in glioblastoma-type brain tumors, but it is not clear how this contributes to tumor growth. Here we show that the siRNA-mediated loss of MELK in U87 MG glioblastoma cells causes a G1/S phase cell cycle arrest accompanied by cell death or a senescence-like phenotype that can be rescued by the expression of siRNA-resistant MELK. This cell cycle arrest is mediated by an increased expression of p21(WAF1/CIP1), an inhibitor of cyclin-dependent kinases, and is associated with the hypophosphorylation of the retinoblastoma protein and the down-regulation of E2F target genes. The increased expression of p21 can be explained by the consecutive activation of ATM (ataxia telangiectasia mutated), Chk2, and p53. Intriguingly, the activation of p53 in MELK-deficient cells is not due to an increased stability of p53 but stems from the loss of MDMX (mouse double minute-X), an inhibitor of p53 transactivation. The activation of the ATM-Chk2 pathway in MELK-deficient cells is associated with the accumulation of DNA double-strand breaks during replication, as demonstrated by the appearance of γH2AX foci. Replication stress in these cells is also illustrated by an increased number of stalled replication forks and a reduced fork progression speed. Our data indicate that glioblastoma cells have elevated MELK protein levels to better cope with replication stress during unperturbed S phase. Hence, MELK inhibitors hold great potential for the treatment of glioblastomas as such or in combination with DNA-damaging therapies.
Keywords: Cell Cycle; DNA Damage; Glioblastoma; MELK; Protein Kinases; Replication Stress; Senescence; p21/WAF1/CIP1.
Figures


References
-
- Beullens M., Vancauwenbergh S., Morrice N., Derua R., Ceulemans H., Waelkens E., Bollen M. (2005) Substrate specificity and activity regulation of protein kinase MELK. J. Biol. Chem. 280, 40003–40011 - PubMed
-
- Badouel C., Körner R., Frank-Vaillant M., Couturier A., Nigg E. A., Tassan J. P. (2006) M-phase MELK activity is regulated by MPF and MAPK. Cell Cycle 8, 883–889 - PubMed
-
- Verlinden L., Eelen G., Beullens I., Van Camp M., Van Hummelen P., Engelen K., Van Hellemont R., Marchal K., De Moor B., Foijer F., Te Riele H., Beullens M., Bollen M., Mathieu C., Bouillon R., Verstuyf A. (2005) Characterization of the condensin component Cnap1 and protein kinase MELK as novel E2F target genes down-regulated by 1,25-dihydroxyvitamin D3. J. Biol. Chem. 280, 37319–37330 - PubMed
-
- Badouel C., Chartrain I., Blot J., Tassan J. P. (2010) Maternal embryonic leucine zipper kinase is stabilized in mitosis by phosphorylation and is partially degraded upon mitotic exit. Exp. Cell Res. 316, 2166–2173 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

