Comparison of intravaginal progesterone gel and intramuscular 17-α-hydroxyprogesterone caproate in luteal phase support
- PMID: 23837065
- PMCID: PMC3702717
- DOI: 10.3892/etm.2013.1049
Comparison of intravaginal progesterone gel and intramuscular 17-α-hydroxyprogesterone caproate in luteal phase support
Abstract
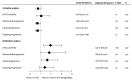
The main objective of this study was to compare the pregnancy rates of intramuscular (IM) 17-α-hydroxyprogesterone caproate (17-HPC) and intravaginal (IV) progesterone gel administration in in vitro fertilization-embryo transfer (IVF-ET) cycles. The IM 17-HPC and IV progesterone groups included 632 (66.4%) and 320 (33.6%) women undergoing the first cycles of IVF-ET treatment, respectively. Multivariate analyses annotated for all potential confounders showed that the use of IV progesterone retained a predictive value for the total β-human chorionic gonadotropin (hCG) positivity and clinical pregnancy rates [adjusted odds ratio (OR), 1.97; 95% confidence interval (CI), 1.28-3.03; P=0.002; and OR, 1.66; 95% CI, 1.07-2.60; P=0.03, respectively]. However, biochemical and on-going pregnancy rates did not differ significantly between the groups (OR, 1.85; 95% CI, 1.00-3.41; P=0.05; and OR, 1.43, 95% CI, 0.89-2.30; P=0.14, respectively). Luteal phase support (LPS) with IV progesterone gel in comparison with IM 17-HPC appears to be associated with higher clinical pregnancy rates in IVF-ET cycles. However, this benefit is clinically irrelevant in terms of on-going pregnancy outcomes.
Keywords: 17-α-hydroxyprogesterone; intravaginal progesterone gel; luteal phase support.
Figures
References
-
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Reproductive Endocrinology and Infertility Progesterone supplementation during the luteal phase and in early pregnancy in the treatment of infertility: an educational bulletin. Fertil Steril. 2008;90(Suppl):S150–S153. - PubMed
-
- Fatemi HM, Popovic-Todorovic B, Papanikolaou E, Donoso P, Devroey P. An update of luteal phase support in stimulated IVF cycles. Hum Reprod Update. 2007;13:581–590. - PubMed
-
- Penzias AS. Luteal phase support. Fertil Steril. 2002;77:318–323. - PubMed
-
- Smitz J, Erard P, Camus M, et al. Pituitary gonadotrophin secretory capacity during the luteal phase in superovulation using GnRH-agonists and HMG in a desensitization or flare-up protocol. Hum Reprod. 1992;7:1225–1229. - PubMed
-
- Albano C, Grimbizis G, Smitz J, et al. The luteal phase of nonsupplemented cycles after ovarian superovulation with human menopausal gonadotropin and the gonadotropin-releasing hormone antagonist Cetrorelix. Fertil Steril. 1998;70:357–359. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources