Pharmacogenomics in early-phase clinical development
- PMID: 23837482
- PMCID: PMC4551460
- DOI: 10.2217/pgs.13.81
Pharmacogenomics in early-phase clinical development
Abstract
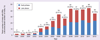
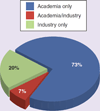
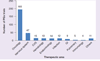
Pharmacogenomics (PGx) offers the promise of utilizing genetic fingerprints to predict individual responses to drugs in terms of safety, efficacy and pharmacokinetics. Early-phase clinical trial PGx applications can identify human genome variations that are meaningful to study design, selection of participants, allocation of resources and clinical research ethics. Results can inform later-phase study design and pipeline developmental decisions. Nevertheless, our review of the clinicaltrials.gov database demonstrates that PGx is rarely used by drug developers. Of the total 323 trials that included PGx as an outcome, 80% have been conducted by academic institutions after initial regulatory approval. Barriers for the application of PGx are discussed. We propose a framework for the role of PGx in early-phase drug development and recommend PGx be universally considered in study design, result interpretation and hypothesis generation for later-phase studies, but PGx results from underpowered studies should not be used by themselves to terminate drug-development programs.
Figures
References
-
- ICH. E15 Definitions for Genomic Biomarkers, Pharmacogenomics, Pharmacogenetics, Genomic Data and Sample Coding Categories. US Department of Health and Human Services, US FDA, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) 2008
-
- Weinshilboum RM, Wang L. Pharmacogenetics and pharmacogenomics: development, science, and translation. Annu. Rev. Genomics Hum. Genet. 2006;7:223–245. - PubMed
-
- Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286(5439):487–491. - PubMed
Website
-
- Clinicaltrials.gov database. www.clinicaltrials.gov.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
